-
PDF
- Split View
-
Views
-
Cite
Cite
Gunnar Klein, Lars Lickfett, Jürgen Schreieck, Thomas Deneke, Marcus Wieczorek, J. Burkowitz, Lourdes Alvarez-Ossorio, Bernd Brüggenjürgen, for the FAST-PVI Study Group, Comparison of ‘anatomically designed’ and ‘point-by-point’ catheter ablations for human atrial fibrillation in terms of procedure timing and costs in German hospitals, EP Europace, Volume 17, Issue 7, July 2015, Pages 1030–1037, https://doi.org/10.1093/europace/euu386
Close - Share Icon Share
Abstract
The purpose of the FAST-PVI study was to compare ‘traditional’ ablation tools based on ‘point-by-point’ technology with the new ‘anatomically designed’ technologies in terms of procedure times and related costs for the treatment of paroxysmal atrial fibrillation.
Four hundred and fifty-two consecutive ablation procedures (222 ‘anatomically designed’, 136 Arctic Front® and 86 PVAC®) and 230 ‘point-by-point’ ablations (100 CARTO XP and 130 NavX navigation systems) performed by nine university centres across Germany from 2006 to 2010 were evaluated retrospectively. Staffing and resources times for each procedure were documented together with patient morbidities, complications, and pulmonary veins isolations. On the basis of DRG data from 2006 to 2010, human resources use and equipment maintenance costs were assigned to ablation procedure and calendar year. All procedural times were significantly higher in ‘point-by-point’ technologies compared with ‘anatomically designed’ ablations [average lab occupancy time 185.30 vs. 280.28 min; physician time 152.21 vs. 238.04 min; support time 183.43 vs. 278.34 min and fluoroscopy time 29.11 vs. 40.72 min; P < 0.001 (95% confidence interval, CI)]. For each ablation procedure human resource use per operating minute resulted in lower costs for ‘anatomically designed’ ablations [€744.24 per patient; P < 0.001 (95% CI)]. Savings due to reduced duration in ‘anatomically designed’ technologies accrued to 20% lower human resource costs. Sensitivity analyses did not lead to any significant variations on the outcomes parameter cost per minute.
FAST-PVI showed reductions in ablation procedural time may lead to increased hospital capacity and non-device-related cost-savings, while maintaining quality.
It has been studied that ‘anatomically designed’ pulmonary vein isolation technologies outperform ‘point-by-point’ procedures in procedural time and can lead to potential capacity and cost-savings.
Study presents a quantification of the procedural costs and benefits.
Hypothesis: ‘Anatomically designed’ technologies induce lower human resource use per operating minute and lower costs for ablations compared with ‘point-by-point’ procedures.
Introduction
Atrial fibrillation (AF) represents the most common sustained arrhythmia, affecting European-wide 5.5% of all subjects above the age of 55 years.1 It impacts adversely on health-related quality of life.2 Atrial fibrillation has been repeatedly shown to be the largest independent risk factor for cardio-embolic stroke.3 Antiarrhythmic drug (AAD) treatment has been proposed as first-line therapy in patients with AF.4
Pulmonary vein isolation (PVI) is a minimally invasive catheter-based ablation technique for treating paroxysmal AF with a risk–benefit ratio sufficient to allow wide-spread adoption.5 Clinical trials have confirmed the value of PVI as a standard treatment for paroxysmal AF patients in whom AADs have failed.6,7 Pulmonary vein isolation has been shown to be an efficient use of healthcare resources in European and North American settings.8,9 The cost reduction is mainly driven by a reduction in AF-related admissions after successful PVI.
Health Technology Assessment agencies increasingly accept the evidence to support PVI in appropriate patient populations.10,11 Nevertheless, some obstacles of current PVI technologies prevent widespread application. First, the experience required to perform the procedure requires sufficiently trained electrophysiologists to meet the demand. Second, the procedure required both special operating theatres as well as ideally hospital facilities offering a backup cardiac surgeon to ensure patient safety.
Recently two circumferential, over-the-wire ablation devices have been introduced: the cryoballoon and the duty cycled bipolar/unipolar radiofrequency (RF) ablation catheters. These tools (Arctic Front®, Medtronic and PVAC®, Medtronic) are pre-shaped to create the PVI lesion and eliminate the need for navigation with the potential to reduce overall procedure time and improve efficiency of the PVI procedure. Both techniques have demonstrated comparable results in terms of efficacy and safety,12 but cryoballoon technology resulted in a lower periprocedural complication rate.13
The FAST-PVI study hypothesized that ‘anatomically designed’ technologies would outperform ‘point-by-point’ technologies in terms of procedural time, which in turn would lead to increased capacity and cost-savings. This hypothesis has been previously studied but no quantification of the procedural cost–benefit has been performed.14,15 Hence, the aim of the study was to compare new and traditional ablation tools in term of procedure times and costs savings derived from shorter procedure time, including provider capacity within existing resource constraints.
Methods
Enrolled centre characteristics and procedures
The FAST-PVI study was a retrospective analysis of 452 PVI procedural records of patients with documented highly symptomatic paroxysmal AF and at least one unsuccessful AAD treatment. Nine mid-size university centres across Germany, with a procedural volume of 200–400 AF ablations annually were selected. To reduce learning-curve-related bias, procedures were only enrolled if the operating team had completed more than 50 procedures at the time of enrollment. Exclusion criteria were prior PVI, severe structural heart disease or enlarged left atria (50 mm or more in parasternal long axis by echocardiography), and moderate or severe valvular heart disease. All patients received transesophageal echocardiography at least 1 day prior to the procedure to exclude left atrial thrombi. Procedures during which equipment failure and other non-patient-related events occurred which may have delayed the procedure, were kept in the sample. Only one cryoablation procedure was interrupted because of a defect cryoconsole; 451 PVIs were completely performed. Enrolled centres were asked to contribute equal number of procedures with ‘anatomically designed’ and ‘point-by-point’ technologies.
Anatomically designed and point-by-point technology characteristics
FAST-PVI compared four different catheter-based ablation technologies, two ‘anatomically designed’ and two ‘point-by-point’ depending on the way by which each technology is used to create the PVI lesion. All PVI procedures were performed in adherence with the ESC Guideline indications.6
Cryoablation
Briefly, patients were treated with a double lumen cryoballoon (Arctic Front, Medtronic), either 23 or 28 mm as appropriate for the size of the pulmonary veins (PVs). Optimum position of the cryoballoon in the pulmonary vein antrum was confirmed by pulmonary vein angiography and verification of vessel occlusion. Cryoablation was applied for 5 min at least two times for each vein. When occlusion was not as desired the wire was changed to a different side branch or position of the balloon or flection of the sheath was changed to ensure better occlusion. In case of persistent conductance into PV either a smaller-sized 23 mm balloon was used or a conventional cryocatheter (Freezor Max, Medtronic) or irrigated RF catheter was used to successfully isolate PVs.
Duty cycled bipolar/unipolar radiofrequency ablation
After single transseptal puncture, a fixed curve long 10 F sheath (Frontier Advance; Medtronic) was inserted into the left atrium (LA) and angiography was performed to delineate all PVs. The pulmonary vein ablation catheter (PVAC) was then advanced through the sheath into the LA to perform mapping and ablation of the targeted PVs followed by a multipolar steerable 6F catheter for differential pacing from several positions. Ablation in the antral region was always initiated in a 4:1 bipolar/unipolar ratio with all electrodes showing local electrical activity being activated to achieve contiguous transmural antral lesions, as far as possible, with a single catheter position. Radiofrequency applications in a 2:1 bipolar/unipolar ratio were applied if PV isolation could not be obtained with the 4:1 setting.
Conventional irrigated-tip radiofrequency ablation
In brief, the following catheters were introduced via the right femoral vein for electrophysiological study: (i) a multipolar catheter was positioned in the coronary sinus; (ii) a circumferential mapping catheter (Lasso, Biosense Webster,) was introduced following transseptal access for verification of PV potential disappearance or dissociation before and after ablation; (iii) a 3.5 or 4 mm irrigated-tip ablation catheter for mapping and ablation was introduced through a steerable transseptal sheath. Selective PV angiography was performed before ablation. Pulmonary vein ablation was performed 0.5–1 cm from the ostium, which was defined on the basis of angiography and the three-dimensional geometry as assessed by Carto (Biosense Webster) or Ensite/NavX (St. Jude Medical). The isolation of all PVs was systematically performed in each patient without attempt to first demonstrate their arrhythmogenicity. The procedural endpoint was the elimination or dissociation of the PV potentials as determined by circumferential mapping.
For all procedures irrespective of the technique used, patients were under conscious sedation. Heparin was administered throughout the procedure to achieve an activated clotting time (ACT) >300 s and ACT was checked at least every 30 min. Since duty cycled bipolar/unipolar RF ablation is the most recently introduced AF ablation technique, the absolute number of patients in this group was lower compared with the other techniques.
Procedural records collection selection and patient characteristics
All procedural times were extracted from patient records in a consecutive manner. Procedures performed for indications different than paroxysmal AF or with other ablation procedure different to PVI were excluded. In every centre included, at least one procedure was observed in real time to ensure process comparability and understand record keeping accuracy and practices. These procedures, however, were not included in our sample to avoid selection bias.
Key patient baseline characteristics as left atrial (LA) size, ejection fraction (EF), presence of patent foramen oval (PFO), diabetes, hypertension or coronary artery diseases (CADs), and previous AAD medication were compared with ensure no statistically significant differences existed in the sample. In addition, the number of targeted and isolated PVs per patient and procedure was collected for success evaluation. Non-patient-related adverse events due to ablation procedure were also documented. Data collection and extraction were performed by an external Clinical Research Organization (CRO). Collected procedural records were audited both by the CRO and one member of hospital staff to maximize accuracy and consistency. The consolidated data were then provided and analysed.
Procedural time definitions
Four different procedural times were documented: Lab occupancy time was defined as the time the operating room remains occupied by the patient or the operating team and thus unavailable for either uses. Primary physician time was defined as the time the lead operating physician is required to be present in the operating room and thus is unable to engage in other activities. Support time was defined as the equivalent time for the supporting team, which included nursing and other involved hospital staff except the operating physician. Finally, fluoroscopy time was defined as the cumulative time fluoroscopy was used during a procedure.
Costing inputs
Costs are based on German annual hospital costing data from InEK covering details for human resource and equipment maintenance costs.16 With procedures being performed from 2006 to 2010, corresponding average human resource (hospital staff to deliver procedure) and equipment maintenance cost per catheter ablation procedure per calendar year were assigned. Given that the new ‘anatomically designed’ technologies are on the market since 2009, only the cost inputs from 2009 and 2010 were assigned for these procedures. Fixed general average ablation material costs were not included in the economic evaluation, as they were considered similar for all procedures. Complications were of low severity in nature and assumed to be reflected in costing of procedure time.
The cost inputs are detailed in Table 1. For ‘point-by-point’ procedures, human and equipment maintenance resource costs were assigned as €1036, €1552, €1992, €1687, and €1831 for the years 2006, 2007, 2008, 2009, and 2010, respectively, whereas for the ‘anatomically designed’ procedures, costs of €1328 and €1626 were allocated for the calendar years 2009 and 2010, respectively.
Human sources and equipment maintenance cost allocation based on German DRGsa
| Procedure . | Point-by-point technologies (€) . | Anatomically designed (€) . | |||||
|---|---|---|---|---|---|---|---|
| Year . | 2006 . | 2007 . | 2008 . | 2009 . | 2010 . | 2009 . | 2010 . |
| Human resources | |||||||
| Medical service | 390.14 | 603.28 | 765.84 | 660.34 | 584.14 | 446.00 | 561.49 |
| Med. technician service | 297.43 | 395.20 | 514.14 | 464.67 | 526.20 | 363.61 | 468.50 |
| Equipment maintenance | |||||||
| Medical infrastructure | 146.80 | 213.76 | 319.22 | 237.61 | 273.70 | 179.25 | 238.20 |
| Non-medical infrastructure | 201.73 | 340.18 | 392.26 | 324.28 | 446.70 | 339.53 | 402.91 |
| Total | 1306.10 | 1552.42 | 1991.46 | 1686.90 | 1830.74 | 1328.39 | 1626.10 |
| Procedure . | Point-by-point technologies (€) . | Anatomically designed (€) . | |||||
|---|---|---|---|---|---|---|---|
| Year . | 2006 . | 2007 . | 2008 . | 2009 . | 2010 . | 2009 . | 2010 . |
| Human resources | |||||||
| Medical service | 390.14 | 603.28 | 765.84 | 660.34 | 584.14 | 446.00 | 561.49 |
| Med. technician service | 297.43 | 395.20 | 514.14 | 464.67 | 526.20 | 363.61 | 468.50 |
| Equipment maintenance | |||||||
| Medical infrastructure | 146.80 | 213.76 | 319.22 | 237.61 | 273.70 | 179.25 | 238.20 |
| Non-medical infrastructure | 201.73 | 340.18 | 392.26 | 324.28 | 446.70 | 339.53 | 402.91 |
| Total | 1306.10 | 1552.42 | 1991.46 | 1686.90 | 1830.74 | 1328.39 | 1626.10 |
Source: INEK (Institut für das Entgeltsystem im Krankenhaus) http://www.g-drg.de.
aPer catheter ablation procedure and calendar year.
Human sources and equipment maintenance cost allocation based on German DRGsa
| Procedure . | Point-by-point technologies (€) . | Anatomically designed (€) . | |||||
|---|---|---|---|---|---|---|---|
| Year . | 2006 . | 2007 . | 2008 . | 2009 . | 2010 . | 2009 . | 2010 . |
| Human resources | |||||||
| Medical service | 390.14 | 603.28 | 765.84 | 660.34 | 584.14 | 446.00 | 561.49 |
| Med. technician service | 297.43 | 395.20 | 514.14 | 464.67 | 526.20 | 363.61 | 468.50 |
| Equipment maintenance | |||||||
| Medical infrastructure | 146.80 | 213.76 | 319.22 | 237.61 | 273.70 | 179.25 | 238.20 |
| Non-medical infrastructure | 201.73 | 340.18 | 392.26 | 324.28 | 446.70 | 339.53 | 402.91 |
| Total | 1306.10 | 1552.42 | 1991.46 | 1686.90 | 1830.74 | 1328.39 | 1626.10 |
| Procedure . | Point-by-point technologies (€) . | Anatomically designed (€) . | |||||
|---|---|---|---|---|---|---|---|
| Year . | 2006 . | 2007 . | 2008 . | 2009 . | 2010 . | 2009 . | 2010 . |
| Human resources | |||||||
| Medical service | 390.14 | 603.28 | 765.84 | 660.34 | 584.14 | 446.00 | 561.49 |
| Med. technician service | 297.43 | 395.20 | 514.14 | 464.67 | 526.20 | 363.61 | 468.50 |
| Equipment maintenance | |||||||
| Medical infrastructure | 146.80 | 213.76 | 319.22 | 237.61 | 273.70 | 179.25 | 238.20 |
| Non-medical infrastructure | 201.73 | 340.18 | 392.26 | 324.28 | 446.70 | 339.53 | 402.91 |
| Total | 1306.10 | 1552.42 | 1991.46 | 1686.90 | 1830.74 | 1328.39 | 1626.10 |
Source: INEK (Institut für das Entgeltsystem im Krankenhaus) http://www.g-drg.de.
aPer catheter ablation procedure and calendar year.
The variable resource cost of an ablation minute was estimated through the sum of the assigned hospital resource costs by ablation weighted according to the four ablation procedures and divided by the average recorded operating time, namely the variable lab occupancy time. Thus, the ablation operating room variable cost per minute was estimated to be €7.41. This value was applied for calculating the human resource cost for each technology.
Statistical methods
Six hundred and twenty-two records were collected of which 170 were excluded from the analysis, leaving 452 in the sample (72.67%). The targeted sample of data are presented as mean ± SE. Baseline characteristics were compared between groups. Dichotomous variables were compared using χ2 tests (2 sides) or Fisher's exact tests, depending on the sample size in each category. Continuous variables were assessed using tests such as the skewness and kurtosis tests and the Shapiro-Wilk tests to control for normal distribution. For not-normally distributed variables, non-parametric tests were used for comparisons between groups: the Mann–Whitney U test when comparing two groups and the Kruskal–Wallis test when comparing four groups. All tests of significance were 2 sided, with P < 0.05 considered statistically significant [95% confidence interval (CI)].
In addition, a univariate analysis of variance (ANOVA) model was generated to adjust for variables as patient age, gender, previous AAD therapy, hypertension, PFO, CAD, EF, LA size, on the dependent variable cost related to procedure time (CI 95%, P < 0.005). Levene test was performed to evaluate the homogeneity of variances test of homogeneity of variances. All tests of significance were 2-sided. The analyses were conducted by using SPSS version 18 (IBM).
Results
Baseline characteristics
Data of 452 patients were collected between 2006 and 2010: 222 related to ‘anatomically designed’ technologies (186 Arctic Front and 86 PVACs) and 230 related to ‘point-by-point’ technologies (100 CARTO XP and 130 NavX navigation systems, respectively). Table 2 summarizes patient baseline characteristics according to the technology modalities. The patients included were about 55–60 years old. Patients in the cryoballoon group and the NavX group were slightly older than patients in PVAC and CARTO groups. However, no statistically significant differences both for age and sex could be observed. Patients in the NavX-group and the cryoballoon group suffered more frequently from hypertension. Weight, EF, incidence of CAD, and diabetes were not significantly different.
Patient baseline characteristics (mean with SD in parenthesis)
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Mean (SD) | ||||||
| Years of age | 56 (11) | 61 (11) | 59 (11) | 56 (10) | 59 (9) | 58 (10) |
| LA size in ml | 46.15 (6.15) | 41.53 (7.08) | 44.08 (6.96) | 42.63 (6.58) | 44.66 (7.16) | 43.83 (6.98) |
| EF % | 65.44 (6.15) | 64.82 (9.81) | 65.07 (8.56) | 63.49 (8.98) | 65.26 (7.20) | 64.53 (8.01) |
| Years on AAD | 5.21 (6.83) | 6.36 (5.92) | 5.88 (6.31) | 5.48 (4.91) | 6.00(6.94) | 5.75 (6.03) |
| Targeted PVs | 3.99 (0.11) | 3.99 (0.43) | 3.99 (0.35) | 3.99 (0.28) | 4.00 (0.15) | 4 (0.22) |
| Isolated PVs | 3.99 (0.11) | 3.89 (0.43) | 3.93 (0.35) | 3.94 (0.28) | 3.98 (0.15) | 3.96 (0.22) |
| Percentage of patients | ||||||
| Male | 62 | 56 | 58 | 62 | 60 | 61 |
| Complications | 7 | 8 | 8 | 10 | 8 | 9 |
| Hypertension | 51 | 57 | 55 | 55 | 70 | 64 |
| AD | 21 | 19 | 20 | 21 | 26 | 24 |
| Diabetes | 6 | 5 | 5 | 7 | 6 | 6 |
| PFO | 8 | 16 | 13 | 3 | 1 | 2 |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Mean (SD) | ||||||
| Years of age | 56 (11) | 61 (11) | 59 (11) | 56 (10) | 59 (9) | 58 (10) |
| LA size in ml | 46.15 (6.15) | 41.53 (7.08) | 44.08 (6.96) | 42.63 (6.58) | 44.66 (7.16) | 43.83 (6.98) |
| EF % | 65.44 (6.15) | 64.82 (9.81) | 65.07 (8.56) | 63.49 (8.98) | 65.26 (7.20) | 64.53 (8.01) |
| Years on AAD | 5.21 (6.83) | 6.36 (5.92) | 5.88 (6.31) | 5.48 (4.91) | 6.00(6.94) | 5.75 (6.03) |
| Targeted PVs | 3.99 (0.11) | 3.99 (0.43) | 3.99 (0.35) | 3.99 (0.28) | 4.00 (0.15) | 4 (0.22) |
| Isolated PVs | 3.99 (0.11) | 3.89 (0.43) | 3.93 (0.35) | 3.94 (0.28) | 3.98 (0.15) | 3.96 (0.22) |
| Percentage of patients | ||||||
| Male | 62 | 56 | 58 | 62 | 60 | 61 |
| Complications | 7 | 8 | 8 | 10 | 8 | 9 |
| Hypertension | 51 | 57 | 55 | 55 | 70 | 64 |
| AD | 21 | 19 | 20 | 21 | 26 | 24 |
| Diabetes | 6 | 5 | 5 | 7 | 6 | 6 |
| PFO | 8 | 16 | 13 | 3 | 1 | 2 |
LA, left atrium; EF, ejection fraction; CAD, coronary artery diseases; PFO, patent foramen oval; AAD, anti-arrythmic drug; PVs, pulmonary veins.
Patient baseline characteristics (mean with SD in parenthesis)
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Mean (SD) | ||||||
| Years of age | 56 (11) | 61 (11) | 59 (11) | 56 (10) | 59 (9) | 58 (10) |
| LA size in ml | 46.15 (6.15) | 41.53 (7.08) | 44.08 (6.96) | 42.63 (6.58) | 44.66 (7.16) | 43.83 (6.98) |
| EF % | 65.44 (6.15) | 64.82 (9.81) | 65.07 (8.56) | 63.49 (8.98) | 65.26 (7.20) | 64.53 (8.01) |
| Years on AAD | 5.21 (6.83) | 6.36 (5.92) | 5.88 (6.31) | 5.48 (4.91) | 6.00(6.94) | 5.75 (6.03) |
| Targeted PVs | 3.99 (0.11) | 3.99 (0.43) | 3.99 (0.35) | 3.99 (0.28) | 4.00 (0.15) | 4 (0.22) |
| Isolated PVs | 3.99 (0.11) | 3.89 (0.43) | 3.93 (0.35) | 3.94 (0.28) | 3.98 (0.15) | 3.96 (0.22) |
| Percentage of patients | ||||||
| Male | 62 | 56 | 58 | 62 | 60 | 61 |
| Complications | 7 | 8 | 8 | 10 | 8 | 9 |
| Hypertension | 51 | 57 | 55 | 55 | 70 | 64 |
| AD | 21 | 19 | 20 | 21 | 26 | 24 |
| Diabetes | 6 | 5 | 5 | 7 | 6 | 6 |
| PFO | 8 | 16 | 13 | 3 | 1 | 2 |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Mean (SD) | ||||||
| Years of age | 56 (11) | 61 (11) | 59 (11) | 56 (10) | 59 (9) | 58 (10) |
| LA size in ml | 46.15 (6.15) | 41.53 (7.08) | 44.08 (6.96) | 42.63 (6.58) | 44.66 (7.16) | 43.83 (6.98) |
| EF % | 65.44 (6.15) | 64.82 (9.81) | 65.07 (8.56) | 63.49 (8.98) | 65.26 (7.20) | 64.53 (8.01) |
| Years on AAD | 5.21 (6.83) | 6.36 (5.92) | 5.88 (6.31) | 5.48 (4.91) | 6.00(6.94) | 5.75 (6.03) |
| Targeted PVs | 3.99 (0.11) | 3.99 (0.43) | 3.99 (0.35) | 3.99 (0.28) | 4.00 (0.15) | 4 (0.22) |
| Isolated PVs | 3.99 (0.11) | 3.89 (0.43) | 3.93 (0.35) | 3.94 (0.28) | 3.98 (0.15) | 3.96 (0.22) |
| Percentage of patients | ||||||
| Male | 62 | 56 | 58 | 62 | 60 | 61 |
| Complications | 7 | 8 | 8 | 10 | 8 | 9 |
| Hypertension | 51 | 57 | 55 | 55 | 70 | 64 |
| AD | 21 | 19 | 20 | 21 | 26 | 24 |
| Diabetes | 6 | 5 | 5 | 7 | 6 | 6 |
| PFO | 8 | 16 | 13 | 3 | 1 | 2 |
LA, left atrium; EF, ejection fraction; CAD, coronary artery diseases; PFO, patent foramen oval; AAD, anti-arrythmic drug; PVs, pulmonary veins.
All technologies showed similar effectiveness in terms of pulmonary veins isolation and rate of complications. Pulmonary vein isolation success rate was obtained dividing the number of targeted PVs by the number of isolated PVs; resulting of 98.54% and 98.95% for ‘anatomically designed’ and ‘point-by-point’ devices, respectively. Although the complication rates of anatomically designed technologies were a little higher (8% vs. 9%), neither difference was statistical significant. The most common complication was light pericardial effusion, followed by haematomas, decreased diaphragmatic mobility, and temporal paresis. Patients with PFO were principally treated with anatomically designed technologies (13% vs. 2%). Morbidities as the presence of diabetes, cardiovascular diseases, and hypertension were not significantly different between both groups.
Procedural timings
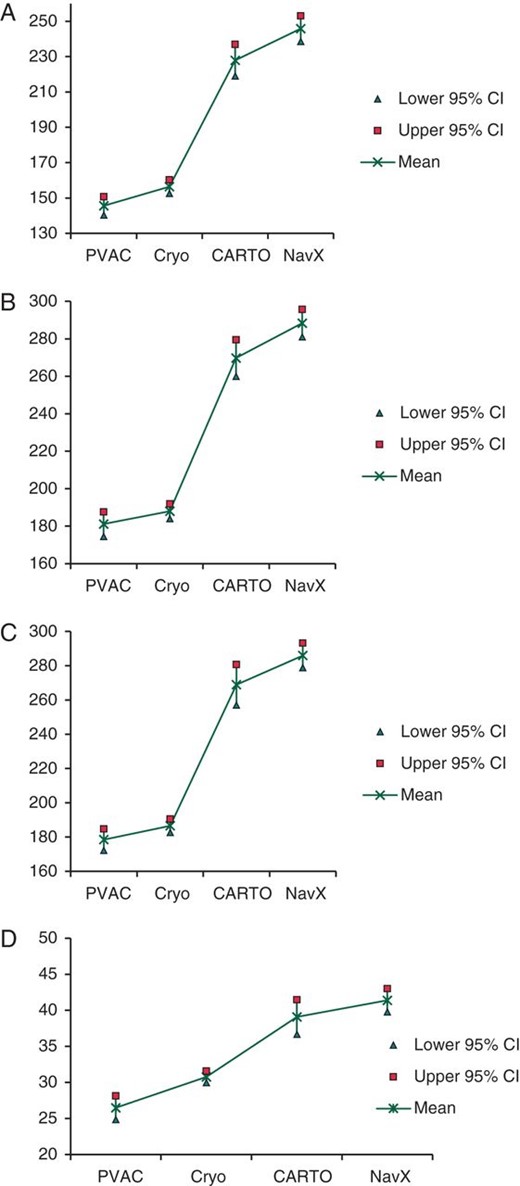
All times (lab occupancy, fluoroscopy, and physician and supporting timing) were significantly longer for patients with ‘point-by-point’ compared with patients treated by the ‘anatomically designed’ methodologies (see Figures 1A–D). The lab occupancy time, namely the time from the patient entering in the operating room to leaving the operating room, was at an average 95 min shorter by the ‘anatomically designed’ ablation procedures in comparison to conventional ‘point-by-point’ procedures. The time from applying local anaesthesia to the patient and removal of the transept sheath (physician time) was also significantly shorter by the ‘anatomically designed’ ablation procedures (86 min). Alike, support and fluoroscopy times were significantly longer by conventional ‘point-by-point’ procedures, 95 and 12 min, respectively. Table 3 summarizes procedural times according to conventional point-by-point and new anatomically designed ablation procedures.
Comparison of mean (SD) mean procedural times in minutes between anatomically designed and point-by-point procedures
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Time differences . |
|---|---|---|---|
| Lab occupancy time | 185.30 (52.34) | 280.28 (89.94) | 94.98* |
| Physician time | 152.21 (45.97) | 238.04 (85.45) | 85.83* |
| Support time | 183.43 (50.69) | 278.34 (88.58) | 94.91* |
| Fluoroscopy time | 29.11 (12.07) | 40.72 (19.31) | 11.61* |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Time differences . |
|---|---|---|---|
| Lab occupancy time | 185.30 (52.34) | 280.28 (89.94) | 94.98* |
| Physician time | 152.21 (45.97) | 238.04 (85.45) | 85.83* |
| Support time | 183.43 (50.69) | 278.34 (88.58) | 94.91* |
| Fluoroscopy time | 29.11 (12.07) | 40.72 (19.31) | 11.61* |
*P < 0.001 (95% CI).
Comparison of mean (SD) mean procedural times in minutes between anatomically designed and point-by-point procedures
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Time differences . |
|---|---|---|---|
| Lab occupancy time | 185.30 (52.34) | 280.28 (89.94) | 94.98* |
| Physician time | 152.21 (45.97) | 238.04 (85.45) | 85.83* |
| Support time | 183.43 (50.69) | 278.34 (88.58) | 94.91* |
| Fluoroscopy time | 29.11 (12.07) | 40.72 (19.31) | 11.61* |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Time differences . |
|---|---|---|---|
| Lab occupancy time | 185.30 (52.34) | 280.28 (89.94) | 94.98* |
| Physician time | 152.21 (45.97) | 238.04 (85.45) | 85.83* |
| Support time | 183.43 (50.69) | 278.34 (88.58) | 94.91* |
| Fluoroscopy time | 29.11 (12.07) | 40.72 (19.31) | 11.61* |
*P < 0.001 (95% CI).
(A) Procedural time—physician time. (B) Procedural time—lab occupancy. (C) Procedural time—support time. (D) Procedural time—fluoroscopy time.
In 65% of the patients treated by PVAC and in 71.5% of patients treated by cryoballoon, the procedure was finished within 200 min, whereas this was only possible in 26.7% and 13.0% of the patients treated by CARTO and Navx conventional point-by-point ablation, respectively.
Costing and cost-savings
The average non-material resource cost for each technology (device costs excluded) corresponded to €1419.74 (688.01), €1472.83 (362.40), €2113.85 (761.06), and €2259.67 (654.15) for PVAC, cryoballoon, CARTO, and Navx procedures, respectively, resulting in a significant cost difference between ‘anatomically designed’ and ‘point-by-point’ procedures of €722.44 (P < 0.001 CI 95%) (Table 4).
Procedure costs in Euro at mean cost-per-minute of lab occupancy time
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Difference anatomically designed vs. point-by-point . | ||||
|---|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | ||
| Mean | 1419.11 | 1472.83 | 1452.03 | 2113.85 | 2259.67 | 2196.27 | −744.24* |
| SD | 476.36 | 362.40 | 410.11 | 761.06 | 654.15 | 704.77 | |
| Upper range | 3056.05 | 2899.33 | 3056.05 | 4584.08 | 4231.46 | 4584.08 | |
| Lower range | 705.24 | 979.50 | 705.24 | 948.16 | 1042.19 | 948.16 | |
| Median | 1367.39 | 1406.57 | 1390.89 | 1974.68 | 2162.74 | 2072.63 | |
| Upper 95% CI | 1521.27 | 1534.28 | 1506.27 | 2264.86 | 2373.18 | 2287.84 | |
| Lower 95%CI | 1317.01 | 1411.37 | 1397.78 | 1962.84 | 2146.16 | 2104.70 | |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Difference anatomically designed vs. point-by-point . | ||||
|---|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | ||
| Mean | 1419.11 | 1472.83 | 1452.03 | 2113.85 | 2259.67 | 2196.27 | −744.24* |
| SD | 476.36 | 362.40 | 410.11 | 761.06 | 654.15 | 704.77 | |
| Upper range | 3056.05 | 2899.33 | 3056.05 | 4584.08 | 4231.46 | 4584.08 | |
| Lower range | 705.24 | 979.50 | 705.24 | 948.16 | 1042.19 | 948.16 | |
| Median | 1367.39 | 1406.57 | 1390.89 | 1974.68 | 2162.74 | 2072.63 | |
| Upper 95% CI | 1521.27 | 1534.28 | 1506.27 | 2264.86 | 2373.18 | 2287.84 | |
| Lower 95%CI | 1317.01 | 1411.37 | 1397.78 | 1962.84 | 2146.16 | 2104.70 | |
SD, Standard deviation.
*P < 0.001 (95% CI).
Procedure costs in Euro at mean cost-per-minute of lab occupancy time
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Difference anatomically designed vs. point-by-point . | ||||
|---|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | ||
| Mean | 1419.11 | 1472.83 | 1452.03 | 2113.85 | 2259.67 | 2196.27 | −744.24* |
| SD | 476.36 | 362.40 | 410.11 | 761.06 | 654.15 | 704.77 | |
| Upper range | 3056.05 | 2899.33 | 3056.05 | 4584.08 | 4231.46 | 4584.08 | |
| Lower range | 705.24 | 979.50 | 705.24 | 948.16 | 1042.19 | 948.16 | |
| Median | 1367.39 | 1406.57 | 1390.89 | 1974.68 | 2162.74 | 2072.63 | |
| Upper 95% CI | 1521.27 | 1534.28 | 1506.27 | 2264.86 | 2373.18 | 2287.84 | |
| Lower 95%CI | 1317.01 | 1411.37 | 1397.78 | 1962.84 | 2146.16 | 2104.70 | |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | Difference anatomically designed vs. point-by-point . | ||||
|---|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | ||
| Mean | 1419.11 | 1472.83 | 1452.03 | 2113.85 | 2259.67 | 2196.27 | −744.24* |
| SD | 476.36 | 362.40 | 410.11 | 761.06 | 654.15 | 704.77 | |
| Upper range | 3056.05 | 2899.33 | 3056.05 | 4584.08 | 4231.46 | 4584.08 | |
| Lower range | 705.24 | 979.50 | 705.24 | 948.16 | 1042.19 | 948.16 | |
| Median | 1367.39 | 1406.57 | 1390.89 | 1974.68 | 2162.74 | 2072.63 | |
| Upper 95% CI | 1521.27 | 1534.28 | 1506.27 | 2264.86 | 2373.18 | 2287.84 | |
| Lower 95%CI | 1317.01 | 1411.37 | 1397.78 | 1962.84 | 2146.16 | 2104.70 | |
SD, Standard deviation.
*P < 0.001 (95% CI).
The ANOVA model adjusted for the following variables: age, gender, LA size, hypertension, CAD, previous ADD therapy, PFO, EF, and ablation procedure. The variables complications, diabetes, and isolation of PV were not included in the model because there were no significant variations across technologies. Non-significant variables were consecutively removed from the model. As expected, the variable ablation procedure was highly significant (P < 0.0001, 95% CI) for all scenarios (Table 5). The model estimated cost per time of €1475.94 and €2285.09 for ‘anatomically designed’ and ‘point-by-point’ procedures, respectively, when all these variables were included and cost per time of €1454.02 and €2227.83 if only the variable EF remained. Our model revealed that no variables lead to statistically significant variations on the dependent variable cost per operating time. Thus, the human resources cost per operating time was considered independent of the observed patient factors and highly dependent on the ablation procedure used.
Mean costs in the ANOVA analysis (dependent variable: ablation procedure costs per intervention time)
| . | Anatomically designed . | Point-by-point . | ||
|---|---|---|---|---|
| Independent variables in model . | Mean (€) . | 95% CI . | Mean (€) . | 95% CI . |
| EF, CAD, AM, Age, LA, PFO HYP, Gender, YAAD | 1475.94 | 1341.24–1610.63 | 2285.09 | 2135.95–2434.82 |
| EF, CAD, AM, Age, LA, PFO HYP | 1519.17 | 1422.74–1615.61 | 2252.63 | 2160.70–2344.56 |
| EF, CAD, AM, Age, LA | 1525.18 | 1431.78–1618.59 | 2210.66 | 2130.52–2290.81 |
| EF, CAD, AM, Age | 1454.89 | 1380.70–1529.08 | 2221.16 | 2145.57–2296.75 |
| EF, CAD, AM | 1456.25 | 1382.10–1530.40 | 2219.75 | 2144.20–2295.29 |
| EF | 1454.20 | 1379.88–1528.51 | 2227.83 | 2152.12–2303.53 |
| . | Anatomically designed . | Point-by-point . | ||
|---|---|---|---|---|
| Independent variables in model . | Mean (€) . | 95% CI . | Mean (€) . | 95% CI . |
| EF, CAD, AM, Age, LA, PFO HYP, Gender, YAAD | 1475.94 | 1341.24–1610.63 | 2285.09 | 2135.95–2434.82 |
| EF, CAD, AM, Age, LA, PFO HYP | 1519.17 | 1422.74–1615.61 | 2252.63 | 2160.70–2344.56 |
| EF, CAD, AM, Age, LA | 1525.18 | 1431.78–1618.59 | 2210.66 | 2130.52–2290.81 |
| EF, CAD, AM, Age | 1454.89 | 1380.70–1529.08 | 2221.16 | 2145.57–2296.75 |
| EF, CAD, AM | 1456.25 | 1382.10–1530.40 | 2219.75 | 2144.20–2295.29 |
| EF | 1454.20 | 1379.88–1528.51 | 2227.83 | 2152.12–2303.53 |
EF, ejection fraction; CAD, coronary artery diseases; AM, ablation method; LA, left atrium size; PFO, patent foramen ovale; HYP, hypertension; YAAD, years with AAD.
Mean costs in the ANOVA analysis (dependent variable: ablation procedure costs per intervention time)
| . | Anatomically designed . | Point-by-point . | ||
|---|---|---|---|---|
| Independent variables in model . | Mean (€) . | 95% CI . | Mean (€) . | 95% CI . |
| EF, CAD, AM, Age, LA, PFO HYP, Gender, YAAD | 1475.94 | 1341.24–1610.63 | 2285.09 | 2135.95–2434.82 |
| EF, CAD, AM, Age, LA, PFO HYP | 1519.17 | 1422.74–1615.61 | 2252.63 | 2160.70–2344.56 |
| EF, CAD, AM, Age, LA | 1525.18 | 1431.78–1618.59 | 2210.66 | 2130.52–2290.81 |
| EF, CAD, AM, Age | 1454.89 | 1380.70–1529.08 | 2221.16 | 2145.57–2296.75 |
| EF, CAD, AM | 1456.25 | 1382.10–1530.40 | 2219.75 | 2144.20–2295.29 |
| EF | 1454.20 | 1379.88–1528.51 | 2227.83 | 2152.12–2303.53 |
| . | Anatomically designed . | Point-by-point . | ||
|---|---|---|---|---|
| Independent variables in model . | Mean (€) . | 95% CI . | Mean (€) . | 95% CI . |
| EF, CAD, AM, Age, LA, PFO HYP, Gender, YAAD | 1475.94 | 1341.24–1610.63 | 2285.09 | 2135.95–2434.82 |
| EF, CAD, AM, Age, LA, PFO HYP | 1519.17 | 1422.74–1615.61 | 2252.63 | 2160.70–2344.56 |
| EF, CAD, AM, Age, LA | 1525.18 | 1431.78–1618.59 | 2210.66 | 2130.52–2290.81 |
| EF, CAD, AM, Age | 1454.89 | 1380.70–1529.08 | 2221.16 | 2145.57–2296.75 |
| EF, CAD, AM | 1456.25 | 1382.10–1530.40 | 2219.75 | 2144.20–2295.29 |
| EF | 1454.20 | 1379.88–1528.51 | 2227.83 | 2152.12–2303.53 |
EF, ejection fraction; CAD, coronary artery diseases; AM, ablation method; LA, left atrium size; PFO, patent foramen ovale; HYP, hypertension; YAAD, years with AAD.
Complications
The intra- and post-intervention complication rates were low (Table 6). Haematoma and small pericardial effusions were seen in some cases. Intra-procedure ST-segment elevation, 50% stenosis of left pulmonary vein were rare. Procedure-related complications comprised difficulty in positioning the catheter or difficulty in transseptal puncture.
Complications by procedure
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Hematoma | 1.1% (1) | 2.2% (3) | 1.8% (4) | 3.0% (3) | 0.8% (1) | 1.8% (4) |
| Pericardial effusion | 0% (0) | 2.2% (3) | 1.4% (3) | 2.0% (2) | 3.9% (5) | 3.0% (7) |
| Neural lesion | 2.3% (2) | 2.2% (3) | 2.2% (5) | 0% (0) | 0% (0) | 0% (0) |
| Procedure related | 3.5% (3) | 1.5% (2) | 2.3% (5) | 5.0% (5) | 3.1% (4) | 3.8% (9) |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Hematoma | 1.1% (1) | 2.2% (3) | 1.8% (4) | 3.0% (3) | 0.8% (1) | 1.8% (4) |
| Pericardial effusion | 0% (0) | 2.2% (3) | 1.4% (3) | 2.0% (2) | 3.9% (5) | 3.0% (7) |
| Neural lesion | 2.3% (2) | 2.2% (3) | 2.2% (5) | 0% (0) | 0% (0) | 0% (0) |
| Procedure related | 3.5% (3) | 1.5% (2) | 2.3% (5) | 5.0% (5) | 3.1% (4) | 3.8% (9) |
Complications by procedure
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Hematoma | 1.1% (1) | 2.2% (3) | 1.8% (4) | 3.0% (3) | 0.8% (1) | 1.8% (4) |
| Pericardial effusion | 0% (0) | 2.2% (3) | 1.4% (3) | 2.0% (2) | 3.9% (5) | 3.0% (7) |
| Neural lesion | 2.3% (2) | 2.2% (3) | 2.2% (5) | 0% (0) | 0% (0) | 0% (0) |
| Procedure related | 3.5% (3) | 1.5% (2) | 2.3% (5) | 5.0% (5) | 3.1% (4) | 3.8% (9) |
| . | Anatomically designed (n = 222) . | Point-by-point (n = 230) . | ||||
|---|---|---|---|---|---|---|
| PVAC (n = 86) . | Cryo (n = 136) . | Anatomically designed . | CARTO (n = 100) . | NavX (n = 130) . | Point-by-point . | |
| Hematoma | 1.1% (1) | 2.2% (3) | 1.8% (4) | 3.0% (3) | 0.8% (1) | 1.8% (4) |
| Pericardial effusion | 0% (0) | 2.2% (3) | 1.4% (3) | 2.0% (2) | 3.9% (5) | 3.0% (7) |
| Neural lesion | 2.3% (2) | 2.2% (3) | 2.2% (5) | 0% (0) | 0% (0) | 0% (0) |
| Procedure related | 3.5% (3) | 1.5% (2) | 2.3% (5) | 5.0% (5) | 3.1% (4) | 3.8% (9) |
Discussion
Pulmonary vein isolation offers paroxysmal AF patients a standardized, safe, and efficacious procedure for preventing disease progression and increasing patient quality of life.17 FAST-PVI is the first study to quantify procedure times for four different technologies used for ablation of paroxysmal AF. Our analyses show that new ‘anatomically designed’ ablation procedures are shorter in procedure time which in turn allows saving in human resources costs. We recorded data from 452 patients for the years 2006–2010 and detected a significant reduction of 95 min in procedure time.
Wilber et al.7 demonstrated an average procedural time with point-by-point technologies of 208 min. Studies, which compared point-to-point with other technologies, documented total procedure lengths of 205 and 224 min for point-to-point PVI.14,15 Duration of procedure is measured usually from transfemoral puncture until disconnection of all PVs. This study reported physician's time spent in the operating theatre, which could result in a slightly longer mean duration of 238 min compared with these former findings. Our results of fluoroscopy times of 40 min in the point-to-point PVI procedures are comparable with durations shown in previous studies. Khaykin et al.15 found a fluoroscopy exposure of 50 min. Bittner et al.14 reported a mean of 35 min. In the study of Wilber et al.7 the mean fluoroscopy time was 49 min. Novel second-generation cryoablation procedures reach median procedure and fluoroscopy time of 174 and 23 min, respectively.18 It indicates potential higher costs savings of anatomical design PVI procedures in terms of non-material resource costs.
A study on anatomically designed catheters reported median procedural times of 170 min for cryoablation.19 Bittner et al.14 showed similar procedure time of mean of 171 min for PVAC. Shorter procedure time was documented in studies of Brunelli et al. (median 154 min) and Beukema et al. (mean 139 min).20,21 Our study results of 152 min for physician and 29 min fluoroscopy are in line with these reported findings. Recent developments in PVI ablation systems and procedural changes demonstrated that they can reduce ‘point-by-point’ and anatomically designed procedure times further.22,23
Pulmonary vein isolation can be regarded as a cost-efficient therapeutic option. Economic evaluation of radiofrequency ablation vs. AAD found higher cost of PVI within a 2 months period after therapy selection from payers' perspective ($10,465 vs. $2,556), but achieved cost neutrality two years after the initial procedure compared with AAD.8 The shorter procedure timing of the anatomical designed technologies can optimize the scarce resource of trained electrophysiologists and operating theatre space in terms of costs and the ability to treat more patients. In fact, the use of cryoballoon technology has been associated with a steep learning curve allowing ablations to be performed by operators with little prior experience. It supports current policies to improve AF management and to reduce current procedural times of 4 h and waiting lists.
Our study has limitations. The study applies a retrospective design which might still result in remaining bias. Although sites with more than 50 completed procedures at the time of enrolment were selected, the small range of ablation practices may impact on study results. The study design did not account for long-term treatment success, i.e. after 6–12 months and costs for potential re-interventions. Since monitoring during follow-up was different between the nine centres involved in the study, data would not be fully representative. With regard to costing, we assumed a cost of €7.41 per operating minute, which is an estimate based on recorded operating time data and the German DRG costing during the period 2006–2010. Device costs could not be obtained. Hence the individual decision maker has to evaluate the relevance of the cost offsets obtained.
Our study reports significant reductions of lab occupancy time and duration of physician, support, and fluoroscopy attributable to the use of ‘anatomically designed’ ablation procedures. We identified potential savings in resource time required and related cost-savings, in particular the time spent in the operating theatre. These new technologies may be associated with additional device costs, however, savings due to reduced duration in ‘anatomically designed’ technologies resulted in 66% of human and theatre-related resources costs compared with point-by-point procedure. In total ‘anatomically designed’ ablations allow for procedure-related cost offsets up to an additional material cost of €744 per PVI intervention in German hospitals. Recent developments in PVI ablation systems and procedural changes were associated with further reductions both in ‘point-by-point’ and anatomically designed procedure times.22,23 This could impair the amount of savings shown in this study. The study did not take into account the explicite differences related to within theatre-related complications as a reflection of these costs in procedure time was assumed. However, the low rate suggests a limited impact on procedure time and costs.
The impact of technical progress in ablation procedures should be a key focus of further economic investigations.
Funding
This work was financed by Medtronic GmbH.
Acknowledgements
The authors thank Lydia Vanter for supporting this work. The authors wish to thank the investigation centres in this study: Department of Clinical Electrophysiology, Division of Cardiovascular Medicine, Hannover Medical School, Germany; Department of Cardiology and Electrophysiology, Hospital Porz am Rhein, Cologne, Germany; Medical Clinic IV, Hospital Karlsruhe, Karlsruhe, Germany; Medical Clinic II, Bergmannsheil, University Hospital, Bochum, Germany; Clinic for Internal Medicine III, Tübingen University Hospital, Tübingen, Germany; Department of Electrophysiology, Kaiser-Wilhelm-Hospital, Duisburg, Germany; Department for Internal Medicine B, Greifswald University Hospital, Greifswald, Germany; Medical Clinic I, SHG-Hospital Völklingen, Völklingen, Germany; Medical Clinic II, Bonn University Hospital, Bonn, Germany.
Conflict of interest: B. Brüggenjürgen is head of the institute for Health Economics at the Steinbeis University Berlin. J. Burkowitz and L. Alvarez-Ossorio are employees of Boston Healthcare Associates International GmbH, who were paid consultants to Medtronic GmbH in connection with the development of this manuscript.
References
- atrial fibrillation
- cardiac ablation
- arctic regions
- cost savings
- fluoroscopy
- germany
- hospital bed capacity
- laboratory
- personnel staffing and scheduling
- pulmonary veins
- technology
- morbidity
- paroxysmal atrial fibrillation
- medical devices
- ablation
- human resource management
- sensitivity analysis
- equipment and supply maintenance