-
PDF
- Split View
-
Views
-
Cite
Cite
Lucas Boersma, Lluís Mont, Alessandro Sionis, Emilio García, Josep Brugada, Value of the implantable loop recorder for the management of patients with unexplained syncope, EP Europace, Volume 6, Issue 1, 2004, Pages 70–76, https://doi.org/10.1016/j.eupc.2003.09.006
Close - Share Icon Share
Abstract
Recurrent syncope often remains unexplained despite extensive multidisciplinary screening. The implantable loop recorder (ILR) may be a tool to define the cardiac arrhythmias underlying syncope.
The study population consisted of 43 consecutive patients with unexplained syncope who underwent extensive cardiological screening and were followed with an ILR. During follow-up, 5 patients had only presyncope, 4 had palpitations, and 15 had a true recurrence of syncope. In all patients with palpitations, 3 with presyncope, and 7 with a recurrence of syncope, the ILR excluded arrhythmias. In the patients with a true recurrence, 1 had symptomatic paroxysmal atrial fibrillation (PAF) treated with drugs, 1 had polymorphic ventricular tachycardia (VT) and received an implantable cardioverter defibrillator (ICD), and 7 had asystole and received a pacemaker. Two patients with presyncope received a pacemaker for Mobitz II block and PAF with brady-tachycardia syndrome. One asymptomatic patient received a pacemaker for significant nocturnal asystole recorded by ILR. Abnormalities in the cardiac screening were observed both in patients with and without syncope, but none of these had a predictive value.
The ILR is a valuable and effective tool to establish an arrhythmic cause for unexplained syncope. The results of head-up tilt testing (HUTT) and electrophysiological study (EPS) are neither sufficiently sensitive nor specific enough in this patient group.
Introduction
Syncope is a recurrent and potentially harmful disability that is frequently encountered by cardiologists and drains health care resources. Early studies [1] have shown that 1 in 4 cases may be neurally mediated and about 1 in 5 patients may have a cardiac disorder. The cardiological screening of a patient with syncope may include electrocardiography, exercise testing, echocardiography, carotid sinus massage, 24-h Holter, head-up tilt test, and electrophysiological study [2] . However, in spite of a detailed and often multidisciplinary investigation, one third of the patients may remain undiagnosed. Recently, an implantable loop recorder (ILR) has been developed for continuous monitoring of the cardiac rhythm to unravel the cause of unexplained syncope. The first experiences with the ILR by Krahn et al. [3] and Nierop et al. [4] have shown that the device was safe and was able to detect cardiac arrhythmias in about 23–42% of patients. The population in these studies was, however, not systematically evaluated prior to the ILR to reach a diagnosis. We therefore evaluated the diagnostic value of the ILR in a population that had undergone extensive cardiological screening for recurrent unexplained syncope.
Methods
Between 1998 and 2001, 43 patients had an ILR implanted in our centres because of recurrent unexplained syncope. The patients were either enroled at our own institutions or referred by an external physician and all had given informed consent. They were made to undergo extensive cardiological screening including a thorough history, evaluation of their medication, physical examination, carotid sinus massage, echocardiography, exercise testing, 24-h Holter, HUTT, and EPS to determine the cause of their syncope. The HUTT protocol included a baseline 30 min tilt at 70°, followed by another 15 min with 1–4 μg/min of isoprenaline if no syncope could be provoked. EPS included measurement of the sinus node recovery time, conduction properties of the atrioventricular node and His–Purkinje system, and programmed electrical stimulation in the right atrium and ventricle. Patients were included if they had at least 3 episodes of syncope in the last 6 months with a negative screening result, the device could be adequately manually activated by the patient and follow-up period was likely to be completed, and implantation of the device was considered technically feasible. A positive HUTT was not considered sufficient to reach a final diagnosis. No patient received additional medication after the device implant.
At the outset, patients received the Reveal Medtronic Inc., Minneapolis, MN, USA 9525 manually activated ILR, whereas later the improved Reveal 9526 with an additional automatic detection algorithm was used. The device was implanted subcutaneously in the electrophysiology laboratory by a cardiologist under local anaesthesia with 2% Xylocaine and oral sedation with 5 mg of diazepam. The ILR was programmed to store the ECG 20 min before to 2 min after the manual activation, while the lower and upper detection thresholds of the automatic device algorithm were set at 40 and 180 beats per minute, respectively. After careful post-surgical evaluation and device training, the patients were discharged from the hospital. The first follow-up was planned 1 month after implant, and patients were asked to present themselves every 3 months and as soon as possible after any event of syncope or presyncope.
Results
Patient characteristics
The characteristics of the patients are given in Table 1 . The mean age was 57 years and approximately half were males. Usually, the patients had been highly symptomatic for a longer period of time (median, 15 months) before the ILR was implanted. Some had suffered trauma to the head (abrasion or concussion) or a fracture due to their syncope. The results of the cardiac workup preceding implantation of the ILR are shown in Table 2 . In all patients, the history, evaluation of medication, physical examination, and carotid sinus massage did not reveal any cause for syncope. In 17 of the 43 patients, echocardiographic abnormalities were observed, while in 2 patients it was not performed for logistical reasons. Three patients had undergone aortic valve replacement, one of whom also received mitral valve replacement, with preserved left ventricular function. Four patients had mild to moderate valvular regurgitation of mitral, aortic, or both valves. Two patients had a history of myocardial infarction with local wall motion abnormalities but the LVEF was >45%. Two patients had a history of dilated cardiomyopathy with significantly depressed left ventricular function. Eight patients had some degree of left ventricular hypertrophy, with 2 of them having severe apical hypertrophy. All these patients received oral anticoagulation, when appropriate, in accordance with international guidelines. None of these disorders was considered to be the direct cause for the recurrent syncope.
Patient characteristics
| Number | 43 |
| Male/female | 21/22 |
| Age | 57 years (17–79) mean |
| Symptom duration | 18 months (3–120) median |
| Symptom frequency | 4 (3–20) median |
| Trauma (head/fracture) | 12 |
| ILR Reveal 9525 (manual) | 17 |
| ILR Reveal-plus 9526 (automatic) | 26 |
| Implant location | |
| Pectoral 13, subcostal 26, submammary 4 | |
| Follow-up time | 18 months (1–18) |
| Number | 43 |
| Male/female | 21/22 |
| Age | 57 years (17–79) mean |
| Symptom duration | 18 months (3–120) median |
| Symptom frequency | 4 (3–20) median |
| Trauma (head/fracture) | 12 |
| ILR Reveal 9525 (manual) | 17 |
| ILR Reveal-plus 9526 (automatic) | 26 |
| Implant location | |
| Pectoral 13, subcostal 26, submammary 4 | |
| Follow-up time | 18 months (1–18) |
Characteristics of the patients who received an implantable loop recorder (ILR). Age is given as average value, symptom duration and frequency as median values. See text for details.
Patient characteristics
| Number | 43 |
| Male/female | 21/22 |
| Age | 57 years (17–79) mean |
| Symptom duration | 18 months (3–120) median |
| Symptom frequency | 4 (3–20) median |
| Trauma (head/fracture) | 12 |
| ILR Reveal 9525 (manual) | 17 |
| ILR Reveal-plus 9526 (automatic) | 26 |
| Implant location | |
| Pectoral 13, subcostal 26, submammary 4 | |
| Follow-up time | 18 months (1–18) |
| Number | 43 |
| Male/female | 21/22 |
| Age | 57 years (17–79) mean |
| Symptom duration | 18 months (3–120) median |
| Symptom frequency | 4 (3–20) median |
| Trauma (head/fracture) | 12 |
| ILR Reveal 9525 (manual) | 17 |
| ILR Reveal-plus 9526 (automatic) | 26 |
| Implant location | |
| Pectoral 13, subcostal 26, submammary 4 | |
| Follow-up time | 18 months (1–18) |
Characteristics of the patients who received an implantable loop recorder (ILR). Age is given as average value, symptom duration and frequency as median values. See text for details.
Cardiac diagnostic workup
| Electrocardiogram abnormal | 8/43 |
| LBBB 3, RBBB + LAFB 2, Brugada Sd. 1, AF 2 | |
| 24-h Holter monitoring abnormal | 5/43 |
| Nocturnal pauses 3, asymptomatic bradycardia 1, nsVT 1 | |
| Echocardiography abnormal | 17/41 |
| LVH 8, DCM 2, old MI 2, valve replacement 2, valve regurgitation 4 | |
| HUT-test abnormal | 7/43 |
| Positive test | |
| Electrophysiological study abnormal | 0/42 |
| Electrocardiogram abnormal | 8/43 |
| LBBB 3, RBBB + LAFB 2, Brugada Sd. 1, AF 2 | |
| 24-h Holter monitoring abnormal | 5/43 |
| Nocturnal pauses 3, asymptomatic bradycardia 1, nsVT 1 | |
| Echocardiography abnormal | 17/41 |
| LVH 8, DCM 2, old MI 2, valve replacement 2, valve regurgitation 4 | |
| HUT-test abnormal | 7/43 |
| Positive test | |
| Electrophysiological study abnormal | 0/42 |
Yield of the diagnostic tests prior to ILR implant. See text for details. LBBB, left bundle branch block; RBBB, right bundle branch block; LAFB, left anterior fascicular block; Sd., syndrome; VT, ventricular tachycardia; ns, non-sustained; LVH, left ventricular hypertrophy; DCM, dilated cardiomyopathy; MI, myocardial infarction; HUT, head-up tilt; ILR, implantable loop recorder.
Cardiac diagnostic workup
| Electrocardiogram abnormal | 8/43 |
| LBBB 3, RBBB + LAFB 2, Brugada Sd. 1, AF 2 | |
| 24-h Holter monitoring abnormal | 5/43 |
| Nocturnal pauses 3, asymptomatic bradycardia 1, nsVT 1 | |
| Echocardiography abnormal | 17/41 |
| LVH 8, DCM 2, old MI 2, valve replacement 2, valve regurgitation 4 | |
| HUT-test abnormal | 7/43 |
| Positive test | |
| Electrophysiological study abnormal | 0/42 |
| Electrocardiogram abnormal | 8/43 |
| LBBB 3, RBBB + LAFB 2, Brugada Sd. 1, AF 2 | |
| 24-h Holter monitoring abnormal | 5/43 |
| Nocturnal pauses 3, asymptomatic bradycardia 1, nsVT 1 | |
| Echocardiography abnormal | 17/41 |
| LVH 8, DCM 2, old MI 2, valve replacement 2, valve regurgitation 4 | |
| HUT-test abnormal | 7/43 |
| Positive test | |
| Electrophysiological study abnormal | 0/42 |
Yield of the diagnostic tests prior to ILR implant. See text for details. LBBB, left bundle branch block; RBBB, right bundle branch block; LAFB, left anterior fascicular block; Sd., syndrome; VT, ventricular tachycardia; ns, non-sustained; LVH, left ventricular hypertrophy; DCM, dilated cardiomyopathy; MI, myocardial infarction; HUT, head-up tilt; ILR, implantable loop recorder.
Follow-up of events
The 43 patients were followed for a median time of 18 months (range 1–33). In some cases, the follow-up period exceeded the maximal life span of the ILR which was then removed. During follow-up, in 19 patients (44%), no complaints or events recurred after ILR implant. In the other 24 patients, a total of 52 events occurred. Nineteen episodes of presyncope occurred in 12 patients, 6 episodes of palpitations in 4 patients, and 26 episodes of syncope in 15 patients. Three of the 4 patients with palpitations also had presyncope, and 4 of the 15 patients with syncope also had presyncope.
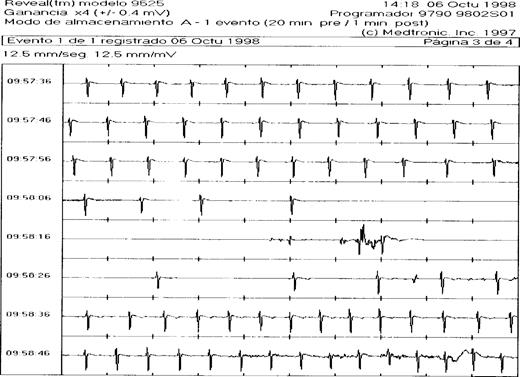
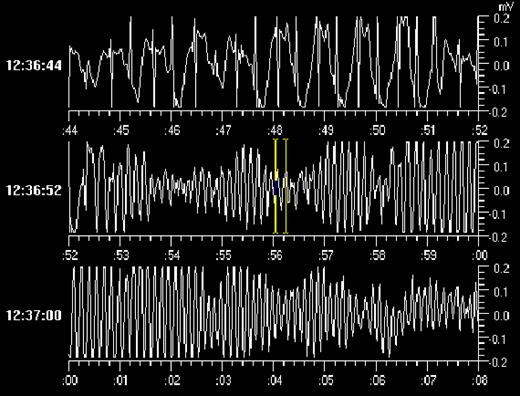
In each patient, the ILR was interrogated at a prescheduled moment or after an event. All the 4 patients with palpitations had episodes of sinus tachycardia recorded during their complaints that did not require treatment. In 2 of the patients with presyncope, the ILR established the arrhythmic cause of syncope as being a symptomatic Mobitz II type AV block in one, and paroxysmal atrial fibrillation with brady-tachycardia syndrome in the other, both treated with a DDD pacemaker. In 9 of the 15 patients with true syncope recurrence, the ILR established an arrhythmia during the event. One had symptomatic paroxysms of atrial fibrillation which were treated with medication. Seven had symptomatic paroxysms of extreme bradycardia to asystole ( Fig. 1 ) which were all treated with a DDD pacemaker. There was 1 patient with syncope in whom the ILR revealed a symptomatic self-limiting episode of polymorphic ventricular tachycardia ( Fig. 2 ) while the baseline ECG had not been diagnostic for long QT or Brugada Syndrome. This patient was equipped with an implantable defibrillator. In the remaining patients with a recurrence, the ILR served to rule out an arrhythmia or conduction disturbance. During syncope, the ILR showed sinus rhythm with rates varying from 60 to 150 beats per minute. Two of these patients were later shown to have epilepsy, while in 3 the diagnosis was psychogenic pseudosyncope. In 2 patients, no definite diagnosis was established. Interestingly, in 1 of the 19 patients who remained asymptomatic during follow-up, routine interrogation of the ILR revealed a significant nocturnal sinus arrest of more than 8 s and this patient received a DDD pacemaker.
ILR registration of syncope due to paroxysmal sinus arrest. ECG registration of 80 s by the implantable loop recorder during episode of syncope. A gradual slowing of sinus rhythm is followed by an asystole of about 15 s before an escape rhythm starts. See text for discussion.
Syncope due to a symptomatic episode of polymorphic VT lasting 2 min in a 16-year-old female patient with a normal QT and no medication. See text for discussion.
In summary, the ILR was able to record an arrhythmic event in 12 of the 43 patients with unexplained syncope who completed their follow-up. In 10 patients, a bradycardiac event was observed that warranted pacemaker implantation; in one, symptomatic atrial fibrillation was found and treated with medication, and in another, polymorphic VT was established and treated with an ICD. The median time to ILR diagnosis of all these patients was 1 month (range 1–12).
Diagnostic value of abnormalities during cardiac screening
Of the 12 patients with an arrhythmic reason for syncope, only 3 had a completely normal workup ( Table 3 ). All others had some abnormalities on ECG, Holter, echocardiography, HUTT, or EPS. However, such abnormalities were also observed in the patients without a recurrence of syncope or arrhythmic events on ILR (compare with Table 2 ). Of the 7 patients with a positive HUTT, only 2 had a recurrence of syncope (29%), compared with 13 of 36 (36%) patients with a negative HUTT. In 8 patients, syncope recurrence was due to asystole which was considered neurally mediated, while the HUTT had been negative in 7 of them. Thus, the response of the patients to HUTT was neither sufficiently sensitive nor specific enough to predict syncope recurrence in this patient population. Two of 8 patients (25%) with an ECG abnormality had recurrence of a syncopal event, compared with 13 of 34 patients (38%) with a normal ECG. Of interest, of the 5 patients with bifascicular AV conduction block, the two with right bundle branch block and left anterior fascicular block (RBBB + LAFB) both had a recurrence of syncope due to asystole. Only 1 of the 5 patients (20%) with an abnormality on Holter had recurrence of a syncopal event, compared with 14 of 38 patients (37%) with a normal Holter. Seven of the 17 patients (41%) with a structural abnormality on echocardiography had a recurrence of a syncopal event, compared with 8 of the 26 patients (31%) with a normal echocardiogram. The EPS was normal in all patients with and without a recurrent syncopal event. No significant abnormalities of the sinus node, AV node or His–Purkinje conduction were detected. Of interest, both the patient with torsades de pointe arrhythmias and the one with a Brugada Syndrome-like ECG were not inducible during EPS. In summary, 14 patients had normal cardiac screening but still in 3 (21%), an arrhythmic cause for recurrent syncope was found. Twenty-nine patients did have abnormalities during their workup, but only 9 (31%) were found to have an underlying arrhythmic cause for syncope.
Diagnostic workup of patients with documented arrhythmic events
| Patient . | Symptom . | Diagnosis . | Therapy . | ECG . | Holter . | Echo . | HUTT . | EPS . |
|---|---|---|---|---|---|---|---|---|
| 1 | Presyncope | PAF with brady-tachy | PM | – | AoR/AoScl + | Neg. | – | |
| 2 | Presyncope | 2° AV block | PM | – | – | Inf. MI | Neg. | – |
| 3 | Syncope | PAF | AAD | – | – | – | Neg. | – |
| 4 | Syncope | Asystole | PM | – | – | AVR/MVR | Neg. | – |
| 5 | Syncope | Asystole | PM | – | Brady<2 s | n.p. | Neg. | – |
| 6 | Syncope | Asystole | PM | RBBB/LAFB | – | LVH + | Pos. | HV 55 ms |
| 7 | Syncope | Asystole | PM | RBBB/LAFB | – | – | Neg. | HV 60 ms |
| 8 | Syncope | Asystole | PM | – | PAF | LVH +, MR + | Neg. | – |
| 9 | Syncope | Asystole | PM | – | AoR/MR +/++ | Neg. | – | |
| 10 | Syncope | Asystole | PM | – | – | – | Neg. | n.p. |
| 11 | Syncope | TdP VT | ICD | – | – | – | Pos. | – |
| 12 | Asymptomatic | Asystole | PM | – | LVH ++ | Neg. | – |
| Patient . | Symptom . | Diagnosis . | Therapy . | ECG . | Holter . | Echo . | HUTT . | EPS . |
|---|---|---|---|---|---|---|---|---|
| 1 | Presyncope | PAF with brady-tachy | PM | – | AoR/AoScl + | Neg. | – | |
| 2 | Presyncope | 2° AV block | PM | – | – | Inf. MI | Neg. | – |
| 3 | Syncope | PAF | AAD | – | – | – | Neg. | – |
| 4 | Syncope | Asystole | PM | – | – | AVR/MVR | Neg. | – |
| 5 | Syncope | Asystole | PM | – | Brady<2 s | n.p. | Neg. | – |
| 6 | Syncope | Asystole | PM | RBBB/LAFB | – | LVH + | Pos. | HV 55 ms |
| 7 | Syncope | Asystole | PM | RBBB/LAFB | – | – | Neg. | HV 60 ms |
| 8 | Syncope | Asystole | PM | – | PAF | LVH +, MR + | Neg. | – |
| 9 | Syncope | Asystole | PM | – | AoR/MR +/++ | Neg. | – | |
| 10 | Syncope | Asystole | PM | – | – | – | Neg. | n.p. |
| 11 | Syncope | TdP VT | ICD | – | – | – | Pos. | – |
| 12 | Asymptomatic | Asystole | PM | – | LVH ++ | Neg. | – |
Results of previous cardiac workup in patients with a recurrence of symptoms or a documented arrhythmia. For abbreviations see Table 2 . PAF, paroxysmal atrial fibrillation; AV, atrioventricular; TdP, torsades de pointe; PM, pacemaker; ICD, implantable cardioverter defibrillator, AoR, aortic regurgitation; AoScl, aortic sclerosis; Inf., inferior; AVR, aortic valve replacement; MVR, mitral valve replacement; brady, bradycardia; MR, mitral regurgitation; HV, His ventricular time; n.p., not performed.
Diagnostic workup of patients with documented arrhythmic events
| Patient . | Symptom . | Diagnosis . | Therapy . | ECG . | Holter . | Echo . | HUTT . | EPS . |
|---|---|---|---|---|---|---|---|---|
| 1 | Presyncope | PAF with brady-tachy | PM | – | AoR/AoScl + | Neg. | – | |
| 2 | Presyncope | 2° AV block | PM | – | – | Inf. MI | Neg. | – |
| 3 | Syncope | PAF | AAD | – | – | – | Neg. | – |
| 4 | Syncope | Asystole | PM | – | – | AVR/MVR | Neg. | – |
| 5 | Syncope | Asystole | PM | – | Brady<2 s | n.p. | Neg. | – |
| 6 | Syncope | Asystole | PM | RBBB/LAFB | – | LVH + | Pos. | HV 55 ms |
| 7 | Syncope | Asystole | PM | RBBB/LAFB | – | – | Neg. | HV 60 ms |
| 8 | Syncope | Asystole | PM | – | PAF | LVH +, MR + | Neg. | – |
| 9 | Syncope | Asystole | PM | – | AoR/MR +/++ | Neg. | – | |
| 10 | Syncope | Asystole | PM | – | – | – | Neg. | n.p. |
| 11 | Syncope | TdP VT | ICD | – | – | – | Pos. | – |
| 12 | Asymptomatic | Asystole | PM | – | LVH ++ | Neg. | – |
| Patient . | Symptom . | Diagnosis . | Therapy . | ECG . | Holter . | Echo . | HUTT . | EPS . |
|---|---|---|---|---|---|---|---|---|
| 1 | Presyncope | PAF with brady-tachy | PM | – | AoR/AoScl + | Neg. | – | |
| 2 | Presyncope | 2° AV block | PM | – | – | Inf. MI | Neg. | – |
| 3 | Syncope | PAF | AAD | – | – | – | Neg. | – |
| 4 | Syncope | Asystole | PM | – | – | AVR/MVR | Neg. | – |
| 5 | Syncope | Asystole | PM | – | Brady<2 s | n.p. | Neg. | – |
| 6 | Syncope | Asystole | PM | RBBB/LAFB | – | LVH + | Pos. | HV 55 ms |
| 7 | Syncope | Asystole | PM | RBBB/LAFB | – | – | Neg. | HV 60 ms |
| 8 | Syncope | Asystole | PM | – | PAF | LVH +, MR + | Neg. | – |
| 9 | Syncope | Asystole | PM | – | AoR/MR +/++ | Neg. | – | |
| 10 | Syncope | Asystole | PM | – | – | – | Neg. | n.p. |
| 11 | Syncope | TdP VT | ICD | – | – | – | Pos. | – |
| 12 | Asymptomatic | Asystole | PM | – | LVH ++ | Neg. | – |
Results of previous cardiac workup in patients with a recurrence of symptoms or a documented arrhythmia. For abbreviations see Table 2 . PAF, paroxysmal atrial fibrillation; AV, atrioventricular; TdP, torsades de pointe; PM, pacemaker; ICD, implantable cardioverter defibrillator, AoR, aortic regurgitation; AoScl, aortic sclerosis; Inf., inferior; AVR, aortic valve replacement; MVR, mitral valve replacement; brady, bradycardia; MR, mitral regurgitation; HV, His ventricular time; n.p., not performed.
Discussion
Syncope due to cardiac arrhythmias
According to the survey by Linzer et al. [1] , 14% of syncopal events are due to cardiac arrhythmias, while 24% have neurally mediated syncope including bradycardia, and a third of the cases remains unsolved. Holter monitoring usually covers only a short time span of 24–48 h, while external event recorders have a limited memory capacity and no detection algorithm so the paroxysmal arrhythmias may be missed. Since the introduction of the Reveal™ ILR, a few investigator groups have published their positive experiences, usually in a multicentre trial, due to difficulties in recruiting patients. In our study, 43 patients with unexplained syncope, including a high percentage with structural heart disease (40%), were followed with an ILR for a longer period of time after extensive cardiac screening. We observed that 35% of patients had a recurrence of syncope of any cause, and 28% of patients were diagnosed as having an arrhythmic event during syncope. The yield of the ILR in providing a diagnosis was somewhat higher than in previous studies by Krahn et al. [3] (25%) and Nierop et al. [4] (23%), probably a small variation due to the patient population. Of interest, the ILR was also able to establish the diagnosis of an arrhythmia in several patients with only presyncope and even in an asymptomatic patient in the absence of a true recurrence. The ILR thus facilitated a diagnosis in this paroxysmal disorder with varying clinical expression as described by Brignole and Menozzi [5] . In our series, we predominantly encountered brady-arrhythmic events during follow-up and only 1 patient with symptomatic PAF. Our discovery of a patient with polymorphic VT must be considered a rare, though fortunate, finding. Krahn et al. [3] and Nierop et al. [4] observed more tachy-arrhythmic events underlying syncope, in 4–11% of cases, respectively. This may be explained by a variation in population, since their patients were not extensively screened prior to the ILR implant.
In all patients with a brady-arrhythmic event during their syncope, we observed a gradual decline in sinus rate followed by asystole as the underlying mechanism. In the recently published ISSUE trial with the ILR, Moya et al. [6] observed that recurrent unexplained syncope in patients without ECG abnormalities or structural heart disease was most often due to sinus arrest. In another patient selection from the ISSUE trial, Brignole et al. [7] showed that 36% of patients with bundle branch block and unexplained syncope had progressive AV conduction block. Our population included 5 patients with bifascicular block and a normal HV time at EPS, but none of them had progressive AV block during follow-up, although the 2 with RBBB + LAFB did have a recurrence of syncope due to asystole. We did observe a case of symptomatic Mobitz II type AV block in a patient with a history of aortic and mitral valve replacement who had a normal ECG and Holter at baseline. However, the documented asystole in our study was usually preceded by a gradual slowing of sinus rhythm and this seems to be most compatible with neurally mediated syncope.
Value and limitations of diagnostic tests
The yield of EPS is highest in patients with known conduction abnormalities or structural cardiac disease. Although in our population we included a substantial number of patients with a structural cardiac abnormality, only 2 had severely depressed LV function, and only 5 had bifascicular AV block. EPS did not provide additional diagnostic information [8] . A recent substudy by the ISSUE investigators [9] showed that patients with severe structural cardiac disease were prone to ventricular arrhythmias but a negative EPS did have recurrences of (pre)syncope similar to their previous findings, however, mostly due to bradycardia or symptomatic atrial fibrillation. By excluding the inducibility of life-threatening tachycardia by EPS, the ILR strategy is safer for the patient [8] .
The HUTT, with or without addition of isoprenaline or nitroglycerine, provides a method to provoke a neurally mediated syncopal event. In our population, only 1 of 7 patients with a positive HUTT had a recurrence of syncope due to asystole. One other patient with a positive HUTT did have a recurrence, but this might have led to a serious misdiagnosis if the ILR had not demonstrated polymorphic VT as the underlying cause for syncope. Considering the fact that the bradycardiac events in our study were most likely neurally mediated or vasovagal, the HUTT was not very useful in reaching a diagnosis in this particular patient population. The HUTT sensitivity was lower than previously described [7] , possibly due to the use of isoprenaline, but in line with the results of Moya et al. [6] . In their population with unexplained syncope without structural heart disease, the rate of recurrence was similar in patients with a positive compared with a negative HUTT.
In our study, 2 of only 5 patients with an abnormality on Holter had a recurrence of syncope. However, only 1 patient with a documented asymptomatic pause of 2 s had a recurrence of syncope due to asystole established by the ILR. Krahn et al. [10] recently also demonstrated that initial ILR monitoring was more likely to provide a diagnosis than initial Holter monitoring in patients without suspected neurally mediated syncope, conduction disturbances or VT, or severe structural heart disease. The present study confirms that the ILR is a better tool to capture the heart rate during syncope. In 28% of patients with unexplained syncope, the ILR established an arrhythmia as the underlying mechanism, while in 16% an arrhythmic cause could be definitely rejected.
Limitations
Although an elaborate screening was planned for each patient, the diagnostic tests were not always completed in every patient. In these patients, the clinical history was not helpful, but one without echocardiography and one without EPS but normal ECG had an asystole on ILR. The outcome of their workup was probably of no consequence for the findings in our study. The follow-up period with a median of 18 months, in some cases, may not have been adequate to establish a diagnosis. On the other hand, in the patients who did have a recurrence of syncope, the median time to diagnosis was only 1 month. Although many patients underwent screening tests by physicians of other specialties, we did not include these data in our study. In our study the patient group was relatively small and the findings may vary when a larger population is examined.
Conclusions
The present study provides additional evidence that unexplained syncope may be related to cardiac arrhythmias in a substantial number of patients. Although the existing cardiac diagnostic tests may provide some clues to the underlying mechanism, the ILR appears to be a relatively easy, safe, and more accurate tool to define cardiac arrhythmias causing unexplained syncope. It provides a significant improvement in the management of this disorder.
Dr. Boersma was supported by a Training Fellowship of the European Society of Cardiology.