-
PDF
- Split View
-
Views
-
Cite
Cite
Kudret Aytemir, Hikmet Yorgun, Uğur Canpolat, M. Levent Şahiner, Ergün Barış Kaya, Banu Evranos, Necla Özer, Initial experience with the TightRail™ Rotating Mechanical Dilator Sheath for transvenous lead extraction, EP Europace, Volume 18, Issue 7, July 2016, Pages 1043–1048, https://doi.org/10.1093/europace/euv245
Close - Share Icon Share
Abstract
In parallel with increasing implantation rates and patients' longer life expectancy, the need for transvenous lead extraction (TLE) as a specialized procedure has shown a significant growth over years. Herein, we aimed to present our initial experience in TLE by using a novel TightRail™ Rotating Mechanical Dilator Sheath.
Between October 2014 and March 2015, a total of 42 leads in 23 patients were removed at our tertiary referral centre. All of the extracted leads were >12 months old and indications for extraction were based on the recommendations of the Heart Rhythm Society. The leads were removed by using the TightRail™ Mechanical Dilator Sheath (Spectranetics Corporation) with the rotational cutting force only. Indications for lead removal included cardiac device infection in 12 (52.2%) cases, lead malfunction in the 10 (43.5%) cases, and upgrade to cardiac resynchronization therapy-defibrillator (CRT-D) in the remaining 1 case (4.3%). The extracted devices were pacemaker in 10 (43.4%) cases, implantable cardioverter-defibrillator (ICD) in 7 (30.4%) cases, and CRT in the remaining 6 (26.0%) subjects. Among 42 leads, 10 (23.8%) were right ventricular, 14 (33.3%) were atrial, 13 (31.0%) were defibrilator, and 5 (11.9%) were coronary sinus electrodes. The median time from implantation was 72 (18–216) months. Complete procedural success with TightRail™ system alone was achieved in 22 (95.7%) patients (41/42 leads) and overall clinical success was 100%. One right ventricular lead was completely removed with the help of femoral snare. All the patients were discharged uneventfully without any complication.
Our preliminary data with small sample size show that TightRail™ Mechanical Dilator Sheath is a new useful tool for chronically implanted pacemaker (PM)/ICD leads. Continued investigation including large patient cohort is required to evaluate success and complication rates in comparison to other tools and techniques.
The TightRail™ Mechanical Dilator Sheath is a novel tool for TLE procedure.
To the best of our knowledge, this is the first observational report in the literature evaluating the efficacy of this new mechanical extraction system in chronically implanted PM/ICD leads.
Use of the TightRail™ system was associated with high success rates of the TLE procedure as a first-line extraction tool without any complication in our small subset of patient population.
Introduction
In parallel with the expanding rate of cardiac implantable electronic devices (CIEDs) implanted worldwide over recent years and increased life expectancy, there is an increasing necessity for safe and efficacious methods to overcome CIED-associated problems including infections, lead dysfunction, lead displacement, and need to upgrade a new technology [e.g. upgrading pacemaker or implantable cardioverter-defibrillator (ICD) to cardiac resynchronization therapy (CRT)].1 Although transvenous lead extraction (TLE) technology has shown significant advancement in the last decade, the procedure requires an experienced interventional team and it is still associated with morbidity and even mortality.2 Therefore, TLE system manufacturers are continuously working on safer and more efficacious devices. A recently developed hand powered device, TightRail™ Mechanical Dilator Sheath (Spectranetics Corp., Colorado Springs, Colorado, USA), has more flexible shaft with a shielded metal blade at the distal tip which has a bidirectional rotating mechanism. The special flexible shaft facilitates forward progression through tortuous vascular structures and frequently encountered fibrotic and calcific attachments. Also dilating metal blade at the distal tip is shielded until activated.
Therefore, in this paper, we aimed to report our initial experience with the TightRail™ system in terms of both safety and efficacy in patients with chronically implanted leads that necessitates extraction.
Methods
Study population
Our Cardiology Clinic is a high volume tertiary referral centre for TLE procedure (>130/years) in our country. The study cohort included a total of 23 patients who underwent TLE procedure in our Electrophysiology Laboratory by using a hand powered rotating mechanical sheath marketed as the TightRail™ system (Spectranetics Corp.) in between October 2014 and March 2015. Data were consecutively collected case-by-case and entered into a computerized database. Patients in whom chronically implanted leads have been removed by manual traction or with a locking stylet were excluded from the study. The indications for lead extraction were based on the recent Heart Rhythm Society's (HRS) recommendations (HRS Expert Consensus Document 2009). Main indications for lead extraction were lead malfunction or infection (pocket infection and/or infective endocarditis). Lead malfunction was defined on the basis of clinically significant alterations in pacing, sensing, and lead impedance. The diagnostic criteria for cardiac device infections that involve the generator pocket, the leads, or both components are described in the recent guidelines.3,4
Informed consent was obtained from each patient before enrolment. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by our local institutional ethics committee.
Lead extraction technique
The TLE procedure was performed in the Electrophysiology Laboratory under deep sedation and local anaesthesia, with invasive blood pressure monitoring via femoral or radial route and non-invasive oxygen saturation monitoring 1,5 and a cardiothoracic surgery team standby. A thorough evaluation of pacemaker (PM)/ICD was performed before the intervention including the assessment of the degree of pacemaker dependency and temporary transvenous pacing was established if necessary. After the skin preparation, the generator pocket was opened and pacemaker generator was disconnected from the leads. The leads were separated from the scar tissue by blunt dissection. Simple manual traction and traction on a lead locking device (Lead Locking Device® EZ, Spectranetics Corp.) with insulation-bound suture was attempted initially. If this systematic approach was unsuccessful, then a TightRail™ Mechanical Dilator Sheath was used as a first choice for both atrial, right ventricular, defibrillator, and/or coronary sinus leads (Figure 1). The TightRail™ Mechanical Dilator Sheath was then positioned over the lead. The operator pulls the handle of the dilator sheath which causes rotation of the cutting blade. The dilator sheath moves along the lead body by cutting fibrous attachments via the distal metal tip. The shielded blade dilates the fibrous attachments by rotating 270° clockwise and 270° counterclockwise with each full trigger activation while extending the blade just 0.5 mm. Once the fibrous attachments are cut, the outer sheath is advanced until another area of attachment is encountered. After the release of leads from fibrous tissue, the leads were pulled back into the sheath and removed (Figures 2 and 3). In case of free floating lead remnants after extraction, femoral or jugular approach with Multisnare (Multi-Snare, PFM, Köln, Germany) was attempted to grasp the remaining part. For patients requiring replacement of their lead, a new lead system was implanted through the same vein in case of lead malfunction or upgrade to a new technology. In case of device infection, the subclavian vein on the opposite side was used after the eradication of infectious microorganism according to the recommendations of current guideline by HRS.1 In PM-dependent patients, reimplantation was performed in the same session if the extraction was due to non-infectious causes. In PM-dependent patients with cardiac device infection, a temporary PM was implanted through the contralateral jugular vein.
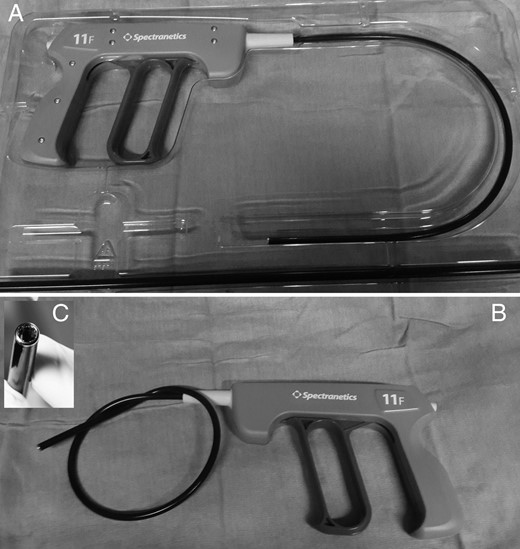
TightRail™ Mechanical Dilator Sheath was illustrated. (A) The system in original pocket including device and outer sheath seperately. (B) The higher flexibility of the TightRail™ shaft and (C) shielded distal metal blade.
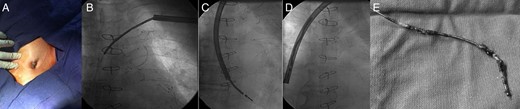
(A) Preprocedural appearance of pacemaker pocket which showing the erosion of ICD generator and lead out of the pocket. (B) Fluoroscopic view of ICD electrode covered by the TightRail™ sheath. (C, D) After the fibrous adhesions were eliminated by the cutting tip of TightRail™ sheath, ICD electrode was pulled back into the sheath and extracted successfully. (E) Fibrous material adherent to defibrillator coil at multiple sites.
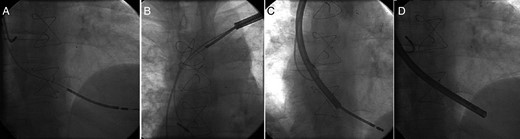
(A) Fluoroscopic view of the atrial and defibrillator leads before extraction of dysfunctional defibrillator lead. (B, C and D) Defibrillator lead was covered by the TightRail™ system and extracted successfully without any damage or wrapping of the coexistent atrial lead.
During the first 48 h after the procedure, continuous non-invasive blood pressure, oxygen saturation, and electrocardiographic monitoring were made and with echocardiographic evaluation just after the intervention and before discharge. At the first month follow-up, a thorough device interrogation was added to the patient assessment with clinical evaluation, electrocardiography, chest X-ray, and transthoracic echocardiography when necessary.
Procedural/clinical success and complications
The success of extraction was determined by means of complete procedural (radiographic) and clinical criteria. Complete procedural success was defined as the removal of all lead components from the vascular space, without any major complication. Clinical success is defined as the removal of all targeted leads and lead material from the vascular space, or retention of a small portion of the lead (<4 cm) that does not negatively impact the outcome goals of the procedure. This may be the tip of the lead or a small part of the lead (conductor coil, insulation, or the latter two combined) when the residual part does not increase the risk of perforation, embolic events, perpetuation of infection or cause any undesired outcome. Complications were defined as major or minor, according to previously published guidelines.1
Statistical analysis
Continuous data are expressed as means ± SD or median (ranges), and all categorical data are expressed as number and percentages. Statistical analysis is performed using SPSS statistical software (version 20; SPSS Inc., Chicago, IL, USA).
Results
Between October 2014 and March 2015, a total of 42 endovascular leads were extracted from 23 patients who admitted to our Cardiology Department. The baseline characteristics of the study population are presented in Table 1. Nineteen patients were male (82.6%) and mean age was 59.1 ± 13.7 (32–79) years. The extracted devices were pacemaker in 10 (43.4%) cases, ICD in 7 (30.4%) cases, and CRT in the remaining 6 (26.0%) subjects. Among 42 leads, 10 (23.8%) were right ventricular, 14 (33.3%) were atrial, 13 (31.0%) were defibrilator, and 5 (11.9%) were coronary sinus electrodes. All ICD leads were of dual coils and the majority of extracted leads had passive fixation mechanism (78.6%). The indications for lead extraction were cardiac device infection in 12 (52.2%) cases, lead malfunction in the 10 (43.5%) cases, and upgrade to CRT-defibrillator (CRT-D) in the remaining 1 case (4.3%).
The baseline demographic and clinical characteristics of the study population (n = 23)
| Parameters . | n = 23 . |
|---|---|
| Clinical variables | |
| Age, years, mean ± SD (range) | 59.1 ± 13.7 (32–79) |
| Male sex, n (%) | 19 (82.6) |
| Body mass index (kg/m2), mean ± SD | 25.4 ± 5.1 |
| Coronary artery disease, n (%) | 11 (47.8) |
| Hypertension, n (%) | 12 (52.2) |
| Diabetes mellitus, n (%) | 6 (26.1) |
| Renal insufficiency, n (%) | 2 (8.7) |
| NYHA Class II–III, n (%) | 11 (47.8) |
| LVEF < 35%, n (%) | 10 (43.5) |
| LVEF (%), mean ± SD | 44.9 ± 18.3 |
| Indications for initial device implantation, n (%) | |
| Dilated cardiomyopathy, syncope, or NSVT | 10 (43.5) |
| Hypertrophic cardiomyopathy, syncope | 1 (4.3) |
| High degree symptomatic AV block | 7 (30.4) |
| Symptomatic bradyarrhythmia | 5 (21.7) |
| Parameters . | n = 23 . |
|---|---|
| Clinical variables | |
| Age, years, mean ± SD (range) | 59.1 ± 13.7 (32–79) |
| Male sex, n (%) | 19 (82.6) |
| Body mass index (kg/m2), mean ± SD | 25.4 ± 5.1 |
| Coronary artery disease, n (%) | 11 (47.8) |
| Hypertension, n (%) | 12 (52.2) |
| Diabetes mellitus, n (%) | 6 (26.1) |
| Renal insufficiency, n (%) | 2 (8.7) |
| NYHA Class II–III, n (%) | 11 (47.8) |
| LVEF < 35%, n (%) | 10 (43.5) |
| LVEF (%), mean ± SD | 44.9 ± 18.3 |
| Indications for initial device implantation, n (%) | |
| Dilated cardiomyopathy, syncope, or NSVT | 10 (43.5) |
| Hypertrophic cardiomyopathy, syncope | 1 (4.3) |
| High degree symptomatic AV block | 7 (30.4) |
| Symptomatic bradyarrhythmia | 5 (21.7) |
AV, atrioventricular; ICD, implantable cardioverter-defibrillator; LVEF, left ventricle ejection fraction; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association.
The baseline demographic and clinical characteristics of the study population (n = 23)
| Parameters . | n = 23 . |
|---|---|
| Clinical variables | |
| Age, years, mean ± SD (range) | 59.1 ± 13.7 (32–79) |
| Male sex, n (%) | 19 (82.6) |
| Body mass index (kg/m2), mean ± SD | 25.4 ± 5.1 |
| Coronary artery disease, n (%) | 11 (47.8) |
| Hypertension, n (%) | 12 (52.2) |
| Diabetes mellitus, n (%) | 6 (26.1) |
| Renal insufficiency, n (%) | 2 (8.7) |
| NYHA Class II–III, n (%) | 11 (47.8) |
| LVEF < 35%, n (%) | 10 (43.5) |
| LVEF (%), mean ± SD | 44.9 ± 18.3 |
| Indications for initial device implantation, n (%) | |
| Dilated cardiomyopathy, syncope, or NSVT | 10 (43.5) |
| Hypertrophic cardiomyopathy, syncope | 1 (4.3) |
| High degree symptomatic AV block | 7 (30.4) |
| Symptomatic bradyarrhythmia | 5 (21.7) |
| Parameters . | n = 23 . |
|---|---|
| Clinical variables | |
| Age, years, mean ± SD (range) | 59.1 ± 13.7 (32–79) |
| Male sex, n (%) | 19 (82.6) |
| Body mass index (kg/m2), mean ± SD | 25.4 ± 5.1 |
| Coronary artery disease, n (%) | 11 (47.8) |
| Hypertension, n (%) | 12 (52.2) |
| Diabetes mellitus, n (%) | 6 (26.1) |
| Renal insufficiency, n (%) | 2 (8.7) |
| NYHA Class II–III, n (%) | 11 (47.8) |
| LVEF < 35%, n (%) | 10 (43.5) |
| LVEF (%), mean ± SD | 44.9 ± 18.3 |
| Indications for initial device implantation, n (%) | |
| Dilated cardiomyopathy, syncope, or NSVT | 10 (43.5) |
| Hypertrophic cardiomyopathy, syncope | 1 (4.3) |
| High degree symptomatic AV block | 7 (30.4) |
| Symptomatic bradyarrhythmia | 5 (21.7) |
AV, atrioventricular; ICD, implantable cardioverter-defibrillator; LVEF, left ventricle ejection fraction; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association.
Re-implantation was performed in 11 (47.8) patients through the left subclavian vein during the same procedure. In one patient, VDD PM was upgraded to biventricular ICD. The median implantation time (insertion to extraction) was 72 (18–216) months. The characteristics of extracted devices and procedure are presented in Table 2.
Explanted device related characteristics of the patients
| Explanted device . | . |
|---|---|
| Number of extracted leads, n | 42 |
| Generator explanted | |
| DDD–PM, n (%) | 6 (26.1) |
| VVI–PM, n (%) | 1 (4.3) |
| VDD–PM, n (%) | 3 (13.0) |
| VVI–ICD, n (%) | 3 (13.0) |
| DDD–ICD, n (%) | 4 (17.4) |
| CRT-D, n (%) | 5 (21.7) |
| CRT-P, n (%) | 1 (4.3) |
| Fixation mechanisms | |
| Active, n (%) | 9 (21.4) |
| Passive, n (%) | 33 (78.6) |
| Median time from implant, months (range) | 72 (18–216) |
| Number of leads per patient | 1.83 ± 0.78 |
| Device re-implanted during same procedure, n (%) | 11 (47.8) |
| 100% pacemaker dependency, n (%) | 10 (43.5) |
| Cardiac leads per patient, n (%) | |
| 1 lead | 9 (39.1) |
| 2 leads | 9 (39.1) |
| 3 leads | 5 (21.7) |
| Indications for removal, n (%) | |
| Pocket erosion and/or infection | 10 (43.5) |
| Endocarditis | 2 (8.7) |
| Lead failure | 10 (43.5) |
| CRT-upgrade | 1 (4.3) |
| Transvenous lead type, n (%) | |
| Atrial | 14 (33.3) |
| Ventricular | 10 (23.8) |
| Defibrillation lead | 13 (31.0) |
| Coronary sinus | 5 (11.9) |
| Procedural details | |
| Total procedure time (min), median (range) | 60 (30–125) |
| Fluoroscopy time (min), median (range) | 5 (1.1–22.4) |
| Dilator sheath diameter | |
| 9 F | 6 (26.1) |
| 11 F | 14 (60.9) |
| 13 F | 3 (13.0) |
| Explanted device . | . |
|---|---|
| Number of extracted leads, n | 42 |
| Generator explanted | |
| DDD–PM, n (%) | 6 (26.1) |
| VVI–PM, n (%) | 1 (4.3) |
| VDD–PM, n (%) | 3 (13.0) |
| VVI–ICD, n (%) | 3 (13.0) |
| DDD–ICD, n (%) | 4 (17.4) |
| CRT-D, n (%) | 5 (21.7) |
| CRT-P, n (%) | 1 (4.3) |
| Fixation mechanisms | |
| Active, n (%) | 9 (21.4) |
| Passive, n (%) | 33 (78.6) |
| Median time from implant, months (range) | 72 (18–216) |
| Number of leads per patient | 1.83 ± 0.78 |
| Device re-implanted during same procedure, n (%) | 11 (47.8) |
| 100% pacemaker dependency, n (%) | 10 (43.5) |
| Cardiac leads per patient, n (%) | |
| 1 lead | 9 (39.1) |
| 2 leads | 9 (39.1) |
| 3 leads | 5 (21.7) |
| Indications for removal, n (%) | |
| Pocket erosion and/or infection | 10 (43.5) |
| Endocarditis | 2 (8.7) |
| Lead failure | 10 (43.5) |
| CRT-upgrade | 1 (4.3) |
| Transvenous lead type, n (%) | |
| Atrial | 14 (33.3) |
| Ventricular | 10 (23.8) |
| Defibrillation lead | 13 (31.0) |
| Coronary sinus | 5 (11.9) |
| Procedural details | |
| Total procedure time (min), median (range) | 60 (30–125) |
| Fluoroscopy time (min), median (range) | 5 (1.1–22.4) |
| Dilator sheath diameter | |
| 9 F | 6 (26.1) |
| 11 F | 14 (60.9) |
| 13 F | 3 (13.0) |
F, French; ICD, implantable cardioverter-defibrillator; CRT-D, biventricular cardioverter defibrillator; CRT-P, biventricular pacemaker.
Explanted device related characteristics of the patients
| Explanted device . | . |
|---|---|
| Number of extracted leads, n | 42 |
| Generator explanted | |
| DDD–PM, n (%) | 6 (26.1) |
| VVI–PM, n (%) | 1 (4.3) |
| VDD–PM, n (%) | 3 (13.0) |
| VVI–ICD, n (%) | 3 (13.0) |
| DDD–ICD, n (%) | 4 (17.4) |
| CRT-D, n (%) | 5 (21.7) |
| CRT-P, n (%) | 1 (4.3) |
| Fixation mechanisms | |
| Active, n (%) | 9 (21.4) |
| Passive, n (%) | 33 (78.6) |
| Median time from implant, months (range) | 72 (18–216) |
| Number of leads per patient | 1.83 ± 0.78 |
| Device re-implanted during same procedure, n (%) | 11 (47.8) |
| 100% pacemaker dependency, n (%) | 10 (43.5) |
| Cardiac leads per patient, n (%) | |
| 1 lead | 9 (39.1) |
| 2 leads | 9 (39.1) |
| 3 leads | 5 (21.7) |
| Indications for removal, n (%) | |
| Pocket erosion and/or infection | 10 (43.5) |
| Endocarditis | 2 (8.7) |
| Lead failure | 10 (43.5) |
| CRT-upgrade | 1 (4.3) |
| Transvenous lead type, n (%) | |
| Atrial | 14 (33.3) |
| Ventricular | 10 (23.8) |
| Defibrillation lead | 13 (31.0) |
| Coronary sinus | 5 (11.9) |
| Procedural details | |
| Total procedure time (min), median (range) | 60 (30–125) |
| Fluoroscopy time (min), median (range) | 5 (1.1–22.4) |
| Dilator sheath diameter | |
| 9 F | 6 (26.1) |
| 11 F | 14 (60.9) |
| 13 F | 3 (13.0) |
| Explanted device . | . |
|---|---|
| Number of extracted leads, n | 42 |
| Generator explanted | |
| DDD–PM, n (%) | 6 (26.1) |
| VVI–PM, n (%) | 1 (4.3) |
| VDD–PM, n (%) | 3 (13.0) |
| VVI–ICD, n (%) | 3 (13.0) |
| DDD–ICD, n (%) | 4 (17.4) |
| CRT-D, n (%) | 5 (21.7) |
| CRT-P, n (%) | 1 (4.3) |
| Fixation mechanisms | |
| Active, n (%) | 9 (21.4) |
| Passive, n (%) | 33 (78.6) |
| Median time from implant, months (range) | 72 (18–216) |
| Number of leads per patient | 1.83 ± 0.78 |
| Device re-implanted during same procedure, n (%) | 11 (47.8) |
| 100% pacemaker dependency, n (%) | 10 (43.5) |
| Cardiac leads per patient, n (%) | |
| 1 lead | 9 (39.1) |
| 2 leads | 9 (39.1) |
| 3 leads | 5 (21.7) |
| Indications for removal, n (%) | |
| Pocket erosion and/or infection | 10 (43.5) |
| Endocarditis | 2 (8.7) |
| Lead failure | 10 (43.5) |
| CRT-upgrade | 1 (4.3) |
| Transvenous lead type, n (%) | |
| Atrial | 14 (33.3) |
| Ventricular | 10 (23.8) |
| Defibrillation lead | 13 (31.0) |
| Coronary sinus | 5 (11.9) |
| Procedural details | |
| Total procedure time (min), median (range) | 60 (30–125) |
| Fluoroscopy time (min), median (range) | 5 (1.1–22.4) |
| Dilator sheath diameter | |
| 9 F | 6 (26.1) |
| 11 F | 14 (60.9) |
| 13 F | 3 (13.0) |
F, French; ICD, implantable cardioverter-defibrillator; CRT-D, biventricular cardioverter defibrillator; CRT-P, biventricular pacemaker.
Complete procedural success using TightRail™ system alone was achieved in 21/22 (95.7%) patients (41/42 leads). In the remaining one ventricular lead (one VDD PM electrode), additional snaring system was required because of the lead fracture. Right jugular venous access was used for snaring and remaining part of ventricular electrode tip was removed successfully. An overall complete procedural success and clinical success were 100%. None of the patients had minor and/or major complications during or at early follow-up. Also we experienced no wrapping of the coexistent leads in two patients with DDD–ICD and CRT-D who underwent extraction of only defibrilator leads.
Discussion
To the best of our knowledge, this is the first observational report in the literature evaluating the initial experience with this new mechanical extraction tool in chronically implanted PM/ICD leads. The use of the TightRail™ system was associated with high success rates of the TLE procedure as a first-line extraction tool without any complication in our small subset of patient population. Of importance, this new system also allowed extraction of defibrillator leads (dual coil) in two patients with DDD–ICD and CRT-D without any wrapping or damage to the neighbouring leads.
Recent years witnessed an increase in the number of CIEDs revision and/or TLEs in parallel with the expanding number of evidence-based indications for device implantation in worldwide.6,7 To ensure optimal patient management, HRS published an expert consensus document.1 Infection of the CIED system including pocket, device, and/or lead is the most common class I indication for lead extraction.4 However, there is an increasing trend in the number of lead dysfunctions because of damage to insulation or wire components or trauma to the leads.8,9 In this preliminary experience with TightRail™ system, we reported that both device infections and lead dysfunction were the most common cause of extraction procedure with approximately similar rates.
The challenges and risks of TLE are principally related to the body's foreign body response to the implanted device at various points. These adhesions not only occur at the lead tip, but are also commonly found along any length of lead where there is contact between the lead and vein, valve or endocardial structures. This response begins at implantation with thrombus formation throughout the lead. Fibrosis of the thrombus occurs after encapsulation of the leads with a fibrin sheath within 4–5 days of implantation.10–12 Fibrous ingrowth, the main obstacle to lead removal, may vary from patient to patient, affecting the complexity, duration, and outcomes of the procedure.13 Although manual traction is an effective technique to remove the leads that have been implanted for <12 months, chronically implanted leads develop fibrous adherences around the surrounding structures and require additional extraction tools. The adhesions may prevent straightforward release as tight scar tissue can withhold the lead during traction. In the recent years, TLE methods and tools have developed significantly. The use of the counter-traction technique by the help of mechanical systems has provided removal of the fibrous and calcific attachments throughout the venous system. The use of lead locking devices enhances tensile properties during traction. However, further techniques like radiofrequency, laser energy, and mechanical dilator sheath systems have also been introduced to facilitate the TLE by cutting the surrounding fibrotic tissue around the lead and reduce the procedural time. In a recent study, Segreti et al.14 reported that dwell time (time interval from implantation to removal), dual-coil ICD technology, and passive fixation mechanism have been found as the independent predictors of the presence of fibrous adharences at various points such as subclavian vein, innominate vein, superior vena cava, and heart. In a recent retrospective study by Starck et al.,15 the laser energy and mechanical approach were compared and the clinical success and cost-effectiveness analysis favoured the mechanical approach rather than laser approach. However, there was no randomized study comparing both laser and mechanical approaches. Although our results are preliminary in this study, the success rates with this novel mechanical system were high as in line with previous TLE studies.16–18 In our patient group, we have extracted 97.6% (41/42 leads) of the indicated chronically implanted PM/ICD/coronary sinus leads (median 72 months) by TightRail™ system alone with an overall success rate of 100% and without any minor or major complication. As previously mentioned, dual-coil ICD, passive fixation mechanism, and higher dwell time were known as significant risk factors for fibrous adharences which make difficult to remove leads only by using simple manual traction or extraction wires. In our study group, ∼31% of the extracted leads were dual-coil ICD, 78.6% of leads have had passive fixation mechanism with a relatively higher dwelling time. Thus, we proceeded with TightRail™ system in all those patients.
The TightRail™ mechanical dilator sheath is a novel tool for TLE procedure. First of all, this system has a flexible shaft which facilitate forward progression in case of tortuous vascular structures due to mostly fibrous attachments especially in older patients. Therefore, this seems to provide an advantage to remain co-axial to the extracted leads and prevent us causing severance and wrapping. Another important novelty regarding this device is the dilating metal blade at the distal tip which was ‘shielded’ until activated. The shielded blade dilates the fibrous attachments after activation by rotating 270° clockwise and 270° counterclockwise with each full trigger activation while extending the blade just 0.5 mm.
In our patient group, we extracted only defibrilator leads in two patients with DDD–ICD and CRT-D in whom there was no severance or wrapping by using this novel system. However, wrapping of the undesired electrodes to the target electrodes is an important problem with mechanical dilator sheaths.18 In addition to several improvements, the shaft of this novel system does not rotate with the distal blade, so outer sheath is optional in this technique. We though that such a property provides advantages in prevention of wrapping of older electrodes. Although our preliminary experience indicated such an advantage of this novel system, large-scale data are required for more clear conclusions relevant to the clinical reflections of those aferomentioned properties unique to the novel system.
Our observational report has some limitations. First, these results should be interpreted in the light of single-centre experience and should not be generalized. This observational report was solely aimed to present our preliminary experience with TightRail™ system as a first line therapy for TLE. However, we do not know yet that it will become a first line therapeutic option. Therefore, multicentre registries are needed to confirm our preliminary results. Also, our paper was not designed to compare the safety or efficacy of TightRail™ system to other TLE tools, especially laser systems. Our patient number is small; large-scale randomized studies are needed for better understanding of the safety and efficacy of this new tool. While TightRail™ system has an advantage of more flexible shaft that follows the curvature of the lead and maintains alignment with the lead, such a property might be disadvantageous in severely calcified lesions.
Conclusions
As a result, preliminary findings of our technical report with a small sample size suggest that TightRail™ Mechanical Dilator Sheath may provide an effective and safe option as a TLE tool based on an integration of technological novelties on an already known mechanics. Larger studies are needed to better assess the safety and efficacy of this new system.
Conflict of interest: none declared.