-
PDF
- Split View
-
Views
-
Cite
Cite
James O. Coffey, Andre d'Avila, Srinivas Dukkipati, Stephan B. Danik, Sandeep R. Gangireddy, Jacob S. Koruth, Marc A. Miller, Solomon J. Sager, Charles A. Eggert, Vivek Y. Reddy, Catheter ablation of scar-related atypical atrial flutter, EP Europace, Volume 15, Issue 3, March 2013, Pages 414–419, https://doi.org/10.1093/europace/eus312
Close - Share Icon Share
Abstract
The aim of the study was to assess the impact of isthmus location of atypical atrial flutters/atrial tachycardias (ATs) on outcomes of catheter ablation. Atrial tachycardias are clinically challenging arrhythmias that can occur in the presence of atrial scar—often due to either cardiac surgery or prior ablation for atrial fibrillation. We previously demonstrated a catheter ablation approach employing rapid multielectrode activation mapping with targeted entrainment manoeuvrs. However, the role that AT isthmus location plays in acute and long-term success of ablation remains uncertain.
Retrospective multicenter analysis of 91 consecutive AT patients undergoing ablation using a systematic four-step approach: (i) high-density activation mapping; (ii) analysis of atrial activation to identify wavefronts of electrical propagation; (iii) targeted entrainment of putative channels; and (iv) irrigated radiofrequency ablation of constrained regions of the circuit. Clinical outcomes, procedural details, and clinical profiles were determined. A total of 171 ATs (1.9 ± 1.0 per patient, 26% septal ATs) were targeted for ablation. The acute success rates were 97 and 77% for patients with either non-septal ATs or septal ATs, respectively (P = 0.0023). Similarly, the long-term success rates were 82 and 67% for patients with either no septal ATs or at least one septal AT, respectively (P = 0.1057). The long-term success rates were 75, 88, and 57% for patients with ATs associated with prior catheter ablation, cardiac surgery or MAZE, and idiopathic atrial scar, respectively.
Catheter ablation of AT can be successfully performed employing a strategy of combined high-density activation and entrainment mapping. Septal ATs are associated with higher rates of acute and long-term recurrences.
Introduction
Catheter ablation has proven to be such a safe and effective approach to the treatment of typical atrial flutter that it is now offered as first-line therapy for this arrhythmia by most electrophysiologists.1,2 While patients with typical atrial flutter can be uniformly treated with ablation at the cavo-tricuspid isthmus (CTI) of the right atrium (RA), atrial flutters that occur subsequent to catheter/surgical ablation of atrial fibrillation or arise in the setting of structural heart disease frequently arise from locations other than the CTI.3–7 These scar-related atypical atrial flutters or atrial tachycardias (ATs) are difficult to manage medically and frequently recur after electrical cardioversion.3–6 On the other hand, such ATs represent a more organized atrial substrate on the continuum between AF and sinus rhythm.4,8–10
We have previously described a catheter ablation strategy which combines rapid high-density electroanatomic mapping acquired with multipolar catheters and targeted entrainment to guide ablation of these arrhythmias.11 Briefly, this approach to AT ablation consists of using an impedance-based mapping system and a multielectrode catheter to map the chamber rapidly during AT, followed by analysis of the patterns of atrial activation to identify wavefronts of electrical propagation. Subsequently, entrainment is performed to differentiate those wavefronts that constitute the AT circuit vs. those that merely represent passive atrial activation. Finally, isthmuses represented by constrained channels of activation are targeted for radiofrequency (RF) catheter ablation.
Using this approach in a series of 17 patients with 41 ATs, 33 of which were mappable and 26 of which persisted long enough for ablation to be attempted, ablation acutely terminated 25 of the 26 ATs for which ablation was attempted.11 While this experience validated this strategy for rapidly defining and eliminating scar-related ATs, there are little long-term clinical outcome data. Accordingly, this manuscript examines the acute and long-term outcomes of 91 consecutive patients undergoing catheter ablation of scar-related atypical atrial flutters in the setting of either prior AF ablation or structural heart disease.
Methods
We performed a retrospective multicentre review of 91 consecutive patients presenting for AT ablation to Massachusetts General Hospital, Boston, MA; University of Miami, Miami, FL; and Mount Sinai Medical Center, New York, NY, between 1 September 2006 and 1 October 2011. All patients were referred for catheter ablation for AT and had a history of either prior catheter ablation for atrial fibrillation, surgical ablation for atrial fibrillation, other cardiac surgery, or idiopathic atrial scarring. Patients with only CTI-dependent flutter were excluded. Patient data were obtained by medical record review, event monitoring, and telephone follow-up. Clinical outcomes, echocardiographic findings, procedural details, and clinical profiles were determined for all patients. The Mount Sinai Hospital and School of Medicine IRB approved data collection and case review.
Mapping and ablation procedure
All procedures were performed under general anaesthesia. For all patients, antiarrhythmic and anticoagulant medications were continued up to the day of the ablation. For patients on warfarin, the goal international normalized ratio was therapeutic (international normalized ratio ≥2) at the time of the procedure. For patients on dabigitran, the morning dose was held on the day of the procedure. Patients underwent pre-procedural computed tomographic (CT) imaging of the left atrium (LA), and a transesophageal echocardiogram was performed on the day of the procedure when clinically indicated. Double transseptal punctures were performed in all patients in whom left-sided AT was suspected (80/91, 88%). In these patients, intravenous unfractionated heparin was administered as a bolus immediately prior to transseptal puncture, followed by a continuous infusion to maintain an activated clotting time of at least 350 s. Intracardiac echocardiography was employed in all cases in which transeptal puncture was performed. Standard catheters used included a decapolar or octapolar catheter in the coronary sinus (CS) and a quadripolar reference catheter placed usually in the non-coronary cusp of the aortic root, or in some cases in the distal CS. A steerable transeptal sheath (Agilis) and a Daig SL-1 sheath (St Jude Medical, St Paul, MN, USA) were used to access the LA. In patients in whom AT was thought or known to be right atrial, a deflectable sheath or an SR-0 sheath (St Jude Medical) was advanced to the RA. Multipolar mapping catheters (circular, spiral, or multispined) were placed through these sheaths into the LA and/or RA.
Using an impedance-based mapping system (Ensite NavX navigation system, St Jude Medical), separate multicomponent geometries of the LA appendage, left superior pulmonary vein (PV), left inferior PV, right superior PV, right inferior PV, and LA body were acquired with the multielectrode mapping catheter. The components were then combined into one structure, forming an electroanatomic map of the LA and PVs. For reference, this map was compared with a three-dimensional CT of the LA, which was obtained in all patients prior to the procedure. When ablation was performed in the RA or CS, separate geometries of these structures were created.
Initially, in all patients with prior left atrial catheter or surgical ablation, all PVs were assessed for reconnection via the circular mapping catheter. In cases in which reconnection was detected, the veins were reisolated, often requiring only a single ablation lesion. If AT was not present at baseline, it was induced with rapid atrial pacing. Subsequently, activation maps were rapidly created using the multielectrode mapping catheter. Rapid activation mapping was enabled by the simultaneous acquisition of activation information from multiple electrodes. Each acquisition is colour coded according to timing of activation and individual points were reviewed only when seeming inconsistent with the bulk of the neighbouring points. Upon completion of the activation map, areas of constrained activation resulting from scars and anatomic barriers were identified as potential critical isthmuses (Figures 1–3). Subsequently, entrainment-pacing manoeuvres were performed from the ablation catheter at sites of interest at a cycle length 20 ms shorter than the tachycardia cycle length (TCL). A site was deemed to be part of the circuit if the post-pacing interval measured from the stimulation artefact to the return atrial electrogram on the ablation catheter was within 20 ms of the TCL.
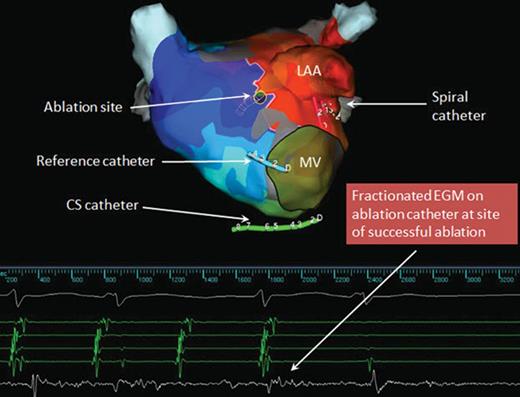
AP activation map showing area of constrained activation where early meets late. Entrainment from this site revealed it to be part of the reentrant tachycardia circuit. The electrogram on the ablation catheter at this site was heavily fractionated. Atrial tachycardia terminated with ablation at that site. After tachycardia termination, several applications are typically delivered around the site of successful termination and/or along a critical isthmus area to ensure durable interruption of the flutter circuits. LAA, left atrial appendage; MV, mitral valve.
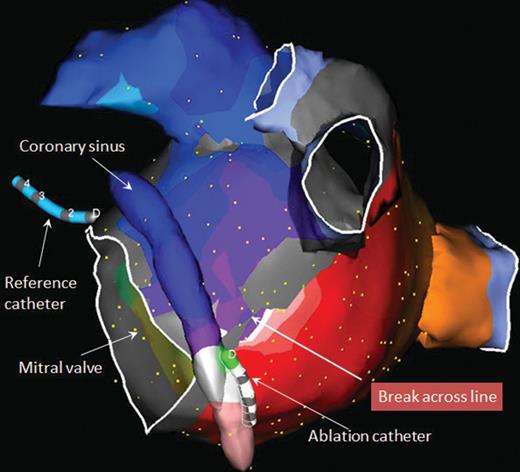
Left anterior oblique view of activation map of mitral isthmus-dependent reentrant atrial tachycardia revealing break across previously created mitral isthmus line. A single ablation lesion delivered from the ablation catheter at this site restored block across the mitral isthmus and terminated the atrial tachycardia. The reference catheter is in the non-coronary cusp of the aortic valve.
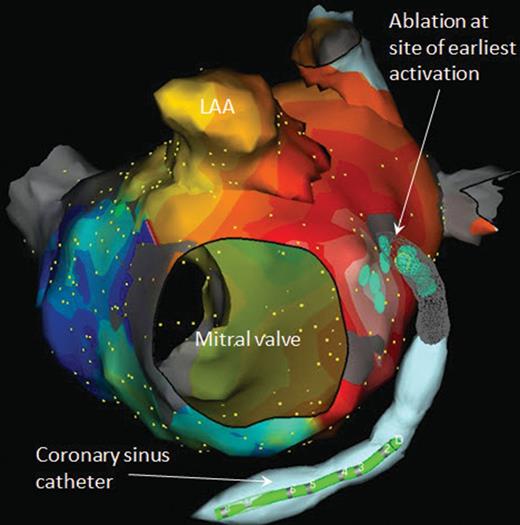
Left anterior oblique view of activation map of peri-mitral focal atrial tachycardia. The map reveals passive activation spreading in all directions from a focal site of earliest activation. Ablation both endocardially and from within the CS was required to terminate this atrial tachycardia. LAA, left atrial appendage.
An irrigated 3.5 mm-tip Celsius Thermo-Cool (Biosense-Webster, Inc., Diamond Bar, CA, USA) ablation catheter was placed through the above-mentioned sheaths into the LA and/or RA and/or CS. The RF generator (Stockert, Biosense Webster) was set to deliver RF energy of up to 35W/43°C in all LA and RA sites excluding the posterior wall of the LA, where a maximum of 25 W was delivered. If the tachycardia terminated during ablation, attempts at re-induction were then performed using programmed atrial stimulation or burst atrial pacing (up to 200 ms). If the tachycardia could not be re-induced, ablation was considered acutely successful. If a new AT was induced either during ablation or during re-induction, it was targeted for ablation using the same strategy as detailed above. In cases in which an AT could not be eliminated via ablation, the patient was cardioverted either electrically (DCCV) or chemically (up to 1.0 mg of IV ibutilide). All patients left the electrophysiology laboratory in sinus rhythm.
Post-procedure management and follow-up
Patients were monitored overnight, and then discharged with instructions to continue anticoagulant and rhythm-control medications that they had been taking before the procedure. Patients were followed in the outpatient clinic and via event monitors. After 3 months, treatment with rhythm-control agents was discontinued in cases with no recurrence of arrhythmia or arrhythmia-related symptoms. Also at this time, warfarin or dabigatran was discontinued and aspirin substituted if appropriate.
Statistical analysis
We applied logistic regression modelling to generate odds ratios (ORs) comparing the acute success rates of patients with non-septal vs. septal ATs. The Cox proportional hazards model was used to estimate hazard ratios (HRs) comparing the long-term success rates of patients with non-septal vs. septal ATs. The Kaplan–Meier method was used to depict graphical assessment of time-related events. Patients without an event or lost to follow-up were censored at the time of their last known event status. We performed all analysis by using STATA software, version 9.2 (Stata Corp., College Station, TX, USA).
Results
The baseline characteristics of the patients are listed in Table 1. Of the 91 patients in this study 59 (65%) had undergone at least one prior catheter ablation, 21 (23%) of which had PV isolation (PVI) alone and the remaining 38 (42%) had additional ablation beyond PVI. Of the 32 patients who had not undergone catheter ablation, 11 (12%) had undergone a prior surgical MAZE procedure, 14 (15%) had other cardiac surgery, and 7 (8%) had idiopathic atrial scar with no history of surgery.
Characteristics and substrate of study patients stratified by the presence of septal atrial tachycardias
| . | All patients (n = 91) . | Septal (n = 30) . | Non-septal (n = 61) . | P value . |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 61 (13; 19–86) | 63 (13; 24–85) | 60 (13; 19–86) | 0.4096 |
| Male | 61 (67%) | 20 (67%) | 41 (67%) | 0.9590 |
| Ejection fraction (%) | 55 (12; 15–73) | 53 (14; 15–68) | 57 (10, 18–73) | 0.1313 |
| Clinical AT cycle length (ms) | 298 (73; 178–680) | 290 (69; 180–459) | 301 (75; 178–680) | 0.0857 |
| Substrate | ||||
| Prior ablation | 59 (65%) | 20 (67%) | 39 (64%) | 0.8002 |
| PVI only | 21 (23%) | 8 (27%) | 13 (21%) | 0.5737 |
| PVI + additional site | 38 (42%) | 12 (40%) | 26 (43%) | 0.8140 |
| Prior Cardiac Surgery | 25 (27%) | 6 (20%) | 19 (31%) | 0.2677 |
| MAZE | 11 (12%) | 3 (10%) | 8 (13%) | 0.6725 |
| Other Cardiac surgery | 14 (15%) | 3 (10%) | 11 (18%) | 0.3235 |
| Idiopathic scar | 7 (8%) | 4 (13%) | 3 (5%) | 0.1602 |
| . | All patients (n = 91) . | Septal (n = 30) . | Non-septal (n = 61) . | P value . |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 61 (13; 19–86) | 63 (13; 24–85) | 60 (13; 19–86) | 0.4096 |
| Male | 61 (67%) | 20 (67%) | 41 (67%) | 0.9590 |
| Ejection fraction (%) | 55 (12; 15–73) | 53 (14; 15–68) | 57 (10, 18–73) | 0.1313 |
| Clinical AT cycle length (ms) | 298 (73; 178–680) | 290 (69; 180–459) | 301 (75; 178–680) | 0.0857 |
| Substrate | ||||
| Prior ablation | 59 (65%) | 20 (67%) | 39 (64%) | 0.8002 |
| PVI only | 21 (23%) | 8 (27%) | 13 (21%) | 0.5737 |
| PVI + additional site | 38 (42%) | 12 (40%) | 26 (43%) | 0.8140 |
| Prior Cardiac Surgery | 25 (27%) | 6 (20%) | 19 (31%) | 0.2677 |
| MAZE | 11 (12%) | 3 (10%) | 8 (13%) | 0.6725 |
| Other Cardiac surgery | 14 (15%) | 3 (10%) | 11 (18%) | 0.3235 |
| Idiopathic scar | 7 (8%) | 4 (13%) | 3 (5%) | 0.1602 |
Characteristics and substrate of study patients stratified by the presence of septal atrial tachycardias
| . | All patients (n = 91) . | Septal (n = 30) . | Non-septal (n = 61) . | P value . |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 61 (13; 19–86) | 63 (13; 24–85) | 60 (13; 19–86) | 0.4096 |
| Male | 61 (67%) | 20 (67%) | 41 (67%) | 0.9590 |
| Ejection fraction (%) | 55 (12; 15–73) | 53 (14; 15–68) | 57 (10, 18–73) | 0.1313 |
| Clinical AT cycle length (ms) | 298 (73; 178–680) | 290 (69; 180–459) | 301 (75; 178–680) | 0.0857 |
| Substrate | ||||
| Prior ablation | 59 (65%) | 20 (67%) | 39 (64%) | 0.8002 |
| PVI only | 21 (23%) | 8 (27%) | 13 (21%) | 0.5737 |
| PVI + additional site | 38 (42%) | 12 (40%) | 26 (43%) | 0.8140 |
| Prior Cardiac Surgery | 25 (27%) | 6 (20%) | 19 (31%) | 0.2677 |
| MAZE | 11 (12%) | 3 (10%) | 8 (13%) | 0.6725 |
| Other Cardiac surgery | 14 (15%) | 3 (10%) | 11 (18%) | 0.3235 |
| Idiopathic scar | 7 (8%) | 4 (13%) | 3 (5%) | 0.1602 |
| . | All patients (n = 91) . | Septal (n = 30) . | Non-septal (n = 61) . | P value . |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 61 (13; 19–86) | 63 (13; 24–85) | 60 (13; 19–86) | 0.4096 |
| Male | 61 (67%) | 20 (67%) | 41 (67%) | 0.9590 |
| Ejection fraction (%) | 55 (12; 15–73) | 53 (14; 15–68) | 57 (10, 18–73) | 0.1313 |
| Clinical AT cycle length (ms) | 298 (73; 178–680) | 290 (69; 180–459) | 301 (75; 178–680) | 0.0857 |
| Substrate | ||||
| Prior ablation | 59 (65%) | 20 (67%) | 39 (64%) | 0.8002 |
| PVI only | 21 (23%) | 8 (27%) | 13 (21%) | 0.5737 |
| PVI + additional site | 38 (42%) | 12 (40%) | 26 (43%) | 0.8140 |
| Prior Cardiac Surgery | 25 (27%) | 6 (20%) | 19 (31%) | 0.2677 |
| MAZE | 11 (12%) | 3 (10%) | 8 (13%) | 0.6725 |
| Other Cardiac surgery | 14 (15%) | 3 (10%) | 11 (18%) | 0.3235 |
| Idiopathic scar | 7 (8%) | 4 (13%) | 3 (5%) | 0.1602 |
Site of origin
A total of 171 mappable ATs (1.9 ± 1.0 ATs per patient) were identified, of which 44 (26%) ATs were of septal origin. Septal flutters were mainly grouped in the following regions: (i) in the lower and more anterior portion of the intra-atrial septum (in front of the right pulmonary veins); (ii) in the region between the RIPV and the coronary sinus ostium (CS os); and (ii) for right-sided flutters, in the vicinity of the CS os. Of the remaining 127 (74%) non-septal ATs, 84 originated from the LA, and 43 originated in the RA. The left atrial ATs were distributed as follows: 39 were related to an LA focus, 30 were classified as mitral isthmus-dependent flutters, and 15 as roof-dependent. Of the right-sided ATs, 31 were related to an RA focus, while 12 were CTI-dependent ATs in patients who had one or more non-CTI-dependent ATs. Patients with only CTI-dependent AT were not included in this analysis.
Acute results
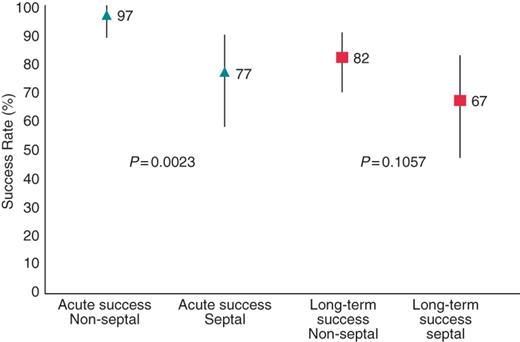
Acute success was defined as termination of tachycardia during ablation and inability to re-induce the same tachycardia. Acute success was measured only in ATs stable enough to be mapped and entrained. A total of 161 ATs (94%) were successfully ablated. Termination of all ATs (‘complete acute success’) with ablation was achieved in 90% of patients [95% confidence interval (CI), 82–95%]. The complete acute success rate was 97% (95% CI, 89–100%) for patients with non-septal ATs and 77% (95% CI, 58–90%) for those with septal ATs (Table 2). Thus, patients with one or more septal AT were less likely to achieve complete acute success than those with non-septal ATs [OR 0.11 (95% CI, 0.02–0.57)].
Acute and long-term success rates by substrate and ablation site
| . | Acute success % (#/n) . | 95% CI . | Long-term success % (#/n) . | 95% CI . |
|---|---|---|---|---|
| All patients | 90 (82/91) | 82–95% | 77 (70/91) | 67–85% |
| Substrate | ||||
| Number of AT sites | ||||
| 1 | 93 (37/40) | 80–98% | 83 (33/40) | 67–93% |
| 2 | 90 (27/30) | 73–98% | 77 (23/30) | 58–90% |
| ≥3 | 86 (18/21) | 64–97% | 67 (14/21) | 43–85% |
| Prior ablation | 88 (52/59) | 77–95% | 75 (44/59) | 62–85% |
| PVI only | 81 (17/21) | 58–95% | 67 (14/21) | 43–85% |
| PVI + additional site | 92 (35/38) | 79–98% | 79 (30/38) | 63–90% |
| Prior cardiac surgery | 96 (24/25) | 80–100% | 88 (22/25) | 69–97% |
| MAZE | 100 (11/11) | 72–100% | 91 (10/11) | 59–100% |
| Other cardiac surgery | 93 (13/14) | 66–100% | 86 (12/14) | 57–98% |
| Idiopathic scar | 86 (6/7) | 42–100% | 57 (4/7) | 18–90% |
| Isthmus | ||||
| Septal | 77 (23/30) | 58–90% | 67 (20/30) | 47–83% |
| Non-septal | 97 (59/61) | 89–100% | 82 (50/61) | 70–91% |
| RA focal | 100 (10/10) | 69–100% | 70 (7/10) | 35–93% |
| LA focal | 82 (9/11) | 48–98% | 82 (9/11) | 48–98% |
| CTI line | 100 (10/10) | 69–100% | 80 (8/10) | 44–97% |
| Roof line | 85 (11/13) | 55–98% | 62 (8/13) | 32–86% |
| Mitral isthmus | 86 (24/28) | 67–96% | 75 (21/28) | 55–89% |
| . | Acute success % (#/n) . | 95% CI . | Long-term success % (#/n) . | 95% CI . |
|---|---|---|---|---|
| All patients | 90 (82/91) | 82–95% | 77 (70/91) | 67–85% |
| Substrate | ||||
| Number of AT sites | ||||
| 1 | 93 (37/40) | 80–98% | 83 (33/40) | 67–93% |
| 2 | 90 (27/30) | 73–98% | 77 (23/30) | 58–90% |
| ≥3 | 86 (18/21) | 64–97% | 67 (14/21) | 43–85% |
| Prior ablation | 88 (52/59) | 77–95% | 75 (44/59) | 62–85% |
| PVI only | 81 (17/21) | 58–95% | 67 (14/21) | 43–85% |
| PVI + additional site | 92 (35/38) | 79–98% | 79 (30/38) | 63–90% |
| Prior cardiac surgery | 96 (24/25) | 80–100% | 88 (22/25) | 69–97% |
| MAZE | 100 (11/11) | 72–100% | 91 (10/11) | 59–100% |
| Other cardiac surgery | 93 (13/14) | 66–100% | 86 (12/14) | 57–98% |
| Idiopathic scar | 86 (6/7) | 42–100% | 57 (4/7) | 18–90% |
| Isthmus | ||||
| Septal | 77 (23/30) | 58–90% | 67 (20/30) | 47–83% |
| Non-septal | 97 (59/61) | 89–100% | 82 (50/61) | 70–91% |
| RA focal | 100 (10/10) | 69–100% | 70 (7/10) | 35–93% |
| LA focal | 82 (9/11) | 48–98% | 82 (9/11) | 48–98% |
| CTI line | 100 (10/10) | 69–100% | 80 (8/10) | 44–97% |
| Roof line | 85 (11/13) | 55–98% | 62 (8/13) | 32–86% |
| Mitral isthmus | 86 (24/28) | 67–96% | 75 (21/28) | 55–89% |
Acute and long-term success rates by substrate and ablation site
| . | Acute success % (#/n) . | 95% CI . | Long-term success % (#/n) . | 95% CI . |
|---|---|---|---|---|
| All patients | 90 (82/91) | 82–95% | 77 (70/91) | 67–85% |
| Substrate | ||||
| Number of AT sites | ||||
| 1 | 93 (37/40) | 80–98% | 83 (33/40) | 67–93% |
| 2 | 90 (27/30) | 73–98% | 77 (23/30) | 58–90% |
| ≥3 | 86 (18/21) | 64–97% | 67 (14/21) | 43–85% |
| Prior ablation | 88 (52/59) | 77–95% | 75 (44/59) | 62–85% |
| PVI only | 81 (17/21) | 58–95% | 67 (14/21) | 43–85% |
| PVI + additional site | 92 (35/38) | 79–98% | 79 (30/38) | 63–90% |
| Prior cardiac surgery | 96 (24/25) | 80–100% | 88 (22/25) | 69–97% |
| MAZE | 100 (11/11) | 72–100% | 91 (10/11) | 59–100% |
| Other cardiac surgery | 93 (13/14) | 66–100% | 86 (12/14) | 57–98% |
| Idiopathic scar | 86 (6/7) | 42–100% | 57 (4/7) | 18–90% |
| Isthmus | ||||
| Septal | 77 (23/30) | 58–90% | 67 (20/30) | 47–83% |
| Non-septal | 97 (59/61) | 89–100% | 82 (50/61) | 70–91% |
| RA focal | 100 (10/10) | 69–100% | 70 (7/10) | 35–93% |
| LA focal | 82 (9/11) | 48–98% | 82 (9/11) | 48–98% |
| CTI line | 100 (10/10) | 69–100% | 80 (8/10) | 44–97% |
| Roof line | 85 (11/13) | 55–98% | 62 (8/13) | 32–86% |
| Mitral isthmus | 86 (24/28) | 67–96% | 75 (21/28) | 55–89% |
| . | Acute success % (#/n) . | 95% CI . | Long-term success % (#/n) . | 95% CI . |
|---|---|---|---|---|
| All patients | 90 (82/91) | 82–95% | 77 (70/91) | 67–85% |
| Substrate | ||||
| Number of AT sites | ||||
| 1 | 93 (37/40) | 80–98% | 83 (33/40) | 67–93% |
| 2 | 90 (27/30) | 73–98% | 77 (23/30) | 58–90% |
| ≥3 | 86 (18/21) | 64–97% | 67 (14/21) | 43–85% |
| Prior ablation | 88 (52/59) | 77–95% | 75 (44/59) | 62–85% |
| PVI only | 81 (17/21) | 58–95% | 67 (14/21) | 43–85% |
| PVI + additional site | 92 (35/38) | 79–98% | 79 (30/38) | 63–90% |
| Prior cardiac surgery | 96 (24/25) | 80–100% | 88 (22/25) | 69–97% |
| MAZE | 100 (11/11) | 72–100% | 91 (10/11) | 59–100% |
| Other cardiac surgery | 93 (13/14) | 66–100% | 86 (12/14) | 57–98% |
| Idiopathic scar | 86 (6/7) | 42–100% | 57 (4/7) | 18–90% |
| Isthmus | ||||
| Septal | 77 (23/30) | 58–90% | 67 (20/30) | 47–83% |
| Non-septal | 97 (59/61) | 89–100% | 82 (50/61) | 70–91% |
| RA focal | 100 (10/10) | 69–100% | 70 (7/10) | 35–93% |
| LA focal | 82 (9/11) | 48–98% | 82 (9/11) | 48–98% |
| CTI line | 100 (10/10) | 69–100% | 80 (8/10) | 44–97% |
| Roof line | 85 (11/13) | 55–98% | 62 (8/13) | 32–86% |
| Mitral isthmus | 86 (24/28) | 67–96% | 75 (21/28) | 55–89% |
Long-term follow-up
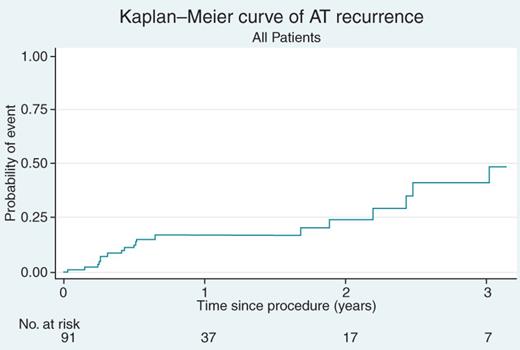
Because all patients left the electrophysiology laboratory in sinus rhythm, we defined recurrence as any post-procedure ATs. Among the 91 patients, there were 21 recurrences, yielding an overall long-term survival free of AT of 77% (95% CI, 67–85%) during a mean follow-up period of 16 ± 12 months (Figure 4). The long term-term success rate for patients with only non-septal ATs was 82% (95% CI, 70–91%), as compared with 67% (95% CI, 47–83%) for patients with one or more septal AT. Thus, AT recurrences were more likely to occur in patients with septal ATs compared with those with non-septal ATs [HR 1.55 (95% CI, 0.63–3.77)] (Figure 5). Patients with more than one AT site were less likely to achieve long-term success. Patients with only one AT site achieved a long-term success rate of 83% (95% CI, 67–93%), while those with two AT sites achieved 77% (95% CI, 58–90%), and those with three or more AT sites achieved only 67% (95% CI, 43–85%).
Kaplan–Meier curve of atrial tachycardia recurrence (all patients).
As shown in Table 2, the baseline substrate underlying the atrial scar—whether prior catheter ablation, surgical ablation, other cardiac surgery, or idiopathic scar—did not have an appreciable effect on the acute success of the ablation procedure. However, the long-term success rate was 88% (95% CI, 69–97%) in patients in whom the prior substrate was surgical scarring (either prior MAZE or other cardiac surgery) as compared with only 57% (95% CI, 18–90%) in those with idiopathic atrial scar. The long-term success after prior catheter ablation was intermediate at 75% (95% CI, 62–85%).
Redo procedures
Of the 21 patients who experienced a recurrence of AT, 9 underwent a repeat procedure, of which 7 (78%) had an acutely successful ablation. Of the remaining 12 patients, 3 underwent successful DCCV, 2 were successfully treated with anti-arrhythmic therapy, and 7 were treated with rate-controlling agents.
Discussion
In this era of rapid growth of catheter ablation as a treatment for AF, post-ablation AT is becoming an increasingly prevalent arrhythmia and represents an interesting and challenging clinical syndrome.12 Particularly subsequent to ablation for persistent AF, in which LA and RA ablation is added to PVI, the occurrence of such arrhythmias is frequent.13–15 Despite general agreement that AT is a relatively organized, often essential, step on the way to achieving durable sinus rhythm in patients with AF or structural heart disease and that catheter ablation should be first-line therapy for such ATs, these arrhythmias remain notable for difficult terminations and high recurrence rates.16 As follow-up to our published short-term results using combined rapid high-density electroanatomic mapping and targeted entrainment, we present this retrospective analysis to further define factors influencing the acute and chronic long-term results of this approach, particularly as it relates to isthmus location.
Main findings of the present study
Our results suggest that the acute and long-term efficacy of catheter ablation of non-CTI-dependent flutters is remarkably good, with an overall success rate of 90% acutely and a long-term survival rate free of flutter of 77%. After a mean of 1.1 procedures, the overall long-term success rate was 85%. However, septal flutters were more difficult to ablate (acute interruption in 97% of non-septal ATs, but in only 77% of septal ATs) and present a higher recurrence rate of 33% as opposed to only 18% for non-septal ATs (Figure 5). The underlying substrate of the AT also influenced the long-term outcome of catheter ablation: patients with ATs in the presence of a prior MAZE procedure fared extremely well with a long-term freedom from AT of over 90%. On the other hand, patients with AT in the setting of idiopathic scar had a high long-term recurrence rate (57%); while this was ultimately not statistically significant, the number of patients in this category was small. Also, as detailed above, patients with more than one AT site were less likely to achieve long-term success. Although such cases are amenable to this approach, the presence of multiple atrial flutters in a given patient suggests the existence of a more complex substrate which may predict a lower rate of acute and long-term success. Finally, for those patients with recurrences who underwent a second ablation procedure for the AT, the success rate of a second procedure was also high at 78%.
Previous studies
Deisenhofer et al.17 prospectively studied 67 patients undergoing circumferential pulmonary vein ablation (CPVA). All patients were screened via continuous 7-day Holter monitoring at 3, 6, and 12 months following the index procedure. Sustained ATs developed in 21 patients, who subsequently underwent second ablation procedures. In 16 patients, 38 mappable ATs (mean 2.4 per patient) were detected. Of note, mapping was performed with an ablation catheter rather than with a multielectrode catheter as in our study. They noted that ablation was acutely successful in 34 of 38 (89%) mapped ATs; however, in only 13 of 21 patients (62%) was sinus rhythm achieved through ablation. This study did not deal with the chronic success of AT ablation using their technique, nor did it address the relevance of isthmus location.
Chae et al.12 assessed 78 post-ablation patients with a total of 155 mappable ATs (mean 2.0 per patient). As with Deisenhofer's study, a multielectrode catheter was not employed for activation mapping. Drawing from a pool of 800 patients undergoing CPVA, 78 patients with 155 ATs were studied. Most ATs (86%) were acutely terminated during ablation; however, only 6 of 16 (38%) of septal ATs could be terminated. During a follow-up period of 13 ± 10 months, recurrence was documented in 30 of the 66 patients (38%). A statistically significant correlation between the presence of septal AT and recurrence was found, as with our patient population.
Jais et al.14 prospectively analysed 128 patients with 238 ATs (mean 1.9 per patient). ATs were mapped using activation and entrainment mapping. As with the other studies, a high-density mapping catheter was not used. The study focused on an approach using cycle length variability to predict focal vs. macrorentrant AT in order to guide ablation. While the rate of acute success was not reported, during a mean follow-up of 21 ± 10 months, sustained AT recurred in 5% of patients, all of whom received a repeat ablation procedure, yielding an ultimate success reported at 95%. No discussion on isthmus location was included in this study.
Our study, therefore, is the first report of long-term results using the combination of rapid high-density electroanatomic mapping acquired with multipolar catheters and targeted entrainment to guide the ablation of these arrhythmias.
Clinical implications
Our results suggest either that the thickness of the inter-atrial septum renders ATs with a septum-dependent circuit relatively resistant to catheter ablation and/or the presence of a more complex circuit that cannot be fully defined with existing mapping techniques are the most likely reason for a less favourable outcome in such patients. Indeed, the septum is composed of the infolded right atrial wall superiorly and inferiorly, and the fibro-fatty ‘sandwich’ of atrial and ventricular musculature anteriorly, and thus is a complex structure which may at times be resistant to current ablation and mapping technologies.18 Septal flutters are likely to be located deeper inside the myocardium and are therefore difficult to reach using current RF ablation technology. As a result, the amount and location of scar (idiopathic or post-procedure) is likely to make ablation in or around these areas more challenging to achieve with unipolar RF applications. In fact, it must be said that, given the complex nature of septal flutter and the inability to completely define the circuit, it remains quite difficult employing currently available techniques to establish with any certainty the definitive mechanisms of septal ATs. Thus, development of alternative or complimentary mapping and ablation techniques in order to achieve higher rates of acute interruption and free-of-flutter long-term results is desirable.
In conclusion, high-density activation mapping combined with selective entrainment mapping offers an efficient and reliable approach to treat non-CTI-dependent ATs occurring after AF ablation, cardiac surgery, or other atrial scar. However, the presence of septal ATs limits the effectiveness of this approach, both acutely and during long-term follow up. New tools or approaches for the mapping and ablation of septal ATs are needed.
Conflict of interest: A.d'A. and V.Y.R. have received consulting fees, speaker honorarium, and grant support from St Jude Medical, the manufacturer of the electroanatomic mapping system, and Biosense Webster, the manufacturer of the ablation catheter used in this series. S.D. has received research grant support from Biosense Webster. C.A.E. is an employee of St Jude Medical. The remaining authors report no conflicts of interest. No external funding was utilized in this research or in the development of this manuscript.
References
- antithymoglobulin
- cardiac arrhythmia
- atrial fibrillation
- atrial flutter
- atrial tachycardia
- cardiac ablation
- cardiac surgery procedures
- atrium
- cicatrix
- follow-up
- treatment outcome
- radiofrequency ablation
- periodic paralysis, potassium-sensitive cardiodysrhythmic type
- ablation
- arterial tortuosity syndrome