-
PDF
- Split View
-
Views
-
Cite
Cite
Erwan Donal, Lionel Giorgis, Serge Cazeau, Christophe Leclercq, Lotfi Senhadji, Amel Amblard, Gael Jauvert, Marc Burban, Alfredo Hernández, Philippe Mabo, Endocardial acceleration (sonR) vs. ultrasound-derived time intervals in recipients of cardiac resynchronization therapy systems, EP Europace, Volume 13, Issue 3, March 2011, Pages 402–408, https://doi.org/10.1093/europace/euq411
Close - Share Icon Share
Abstract
Optimization of cardiac resynchronization therapy (CRT) requires the gathering of cardiac functional information. An accurate timing of the phases of the cardiac cycle is key in the optimization process.
We compared Doppler echocardiography to an automated system, based on the recording of sonR (formerly endocardial acceleration), in the detection of mitral and aortic valves closures and measurements of the duration of systole and diastole. We prospectively studied, under various conditions of cardiac stimulation, 75 recipients of CRT systems (69% men), whose mean age was 72 ± 9.2 years, left ventricular ejection fraction 35 ± 11%, baseline QRS duration 154 ± 29 ms, and New York Heart Association functional class 3.0 ± 0.7. We simultaneously recorded (i) sonR, detected by a non-invasive piezoelectric micro-accelerometer sensor clipped onto an electrode located in the parasternal region, (b) electrocardiogram, and (c) Doppler audio signals, using a multichannel data acquisition and analysis system. The correlation between timing of mitral and aortic valve closure by sonR vs. Doppler signals was examined by linear regression analysis. Correlation coefficients and the average absolute error were calculated. A concordance in the timing of the mitral (r = 0.86, error = 9.7 ms) and aortic (r = 0.93, error = 9.7 ms) valves closure was observed between the two methods in 94% of patients. Similarly, sonR and the Doppler-derived measurements of systolic (r = 0.85, error = 13.4 ms) and diastolic (r = 0.99, error = 12 ms) interval durations were concordant in 80% of patients.
A high concordance was found between sonR and the cardiac ultrasound in the timings of aortic and mitral valve closures and in the estimation of systolic and diastolic intervals durations. These observations suggest that sonR could be used to monitor cardiac function and adaptively optimize CRT systems.
Introduction
Cardiac resynchronization (CRT) is a therapeutic option validated for selected patients suffering from moderate or severe heart failure.1 In previously published trials,2–4 the proportion of non-responders to therapy has been ∼30% in the long term.4,5 Among several factors, including patient selection and lead positioning, the inaccurate programming of the atrioventricular (AV) delay, interventricular (VV) delay, or both, are causes of non-response to CRT. Echocardiography is currently the most reliable method used to (i) estimate the quality of cardiac mechanical synchrony and (ii) programme the optimal AV and VV delays during CRT.6,7 Mechanical and haemodynamic performance needs to be continuously monitored while AV and VV delays are programmed in an orderly sequence.8 However, in clinical practice, this time-consuming, operator-dependent task is not performed systematically and, when performed, is limited to conditions of rest in a supine position.8
We hypothesized that the recently developed sonR™ sensor (Sorin CRM SAS, Clamart, France), which monitors cardiac mechanical function, could, once embedded in an implantable device, (i) facilitate and shorten the CRT optimization procedures, (ii) enable the optimization under various conditions of rest and exercise, and (iii) automatically and adaptively optimize CRT in the long term. This study used an external prototype of the sonR sensor, placed on the patient's chest, to time the closure of the aortic and mitral valves, and compared its performance with echocardiography. Furthermore, since the relative duration of the phases of the cardiac cycle is an important determinant of cardiac mechanical performance,9 we used sonR to monitor the duration of ventricular systole and diastole, with a view to optimize CRT.
Methods
Patient population
The data used for this analysis were collected in prospective registry at three medical centres. Three distinct physicians performed the examinations and each examination was analysed in a dedicated core-Lab (CIC-IT Rennes). We used recordings collected during routine ambulatory visits in 75 patients suffering from chronic heart failure, whose mean age was 72 ± 9 years. All patients had been treated with a CRT system, with or without defibrillator, for a median of 6 months (range 3 days to 102 months). The CRT system was in accordance with guidelines. Patients were all symptomatic [New York Heart Association (NYHA) class III or IV] despite the maximal dosage of angiotensin-converting enzyme-inhibitor and beta-blockers. These patients were all chronically followed in one of the three participating centres and with a dedicated management of their chronic heart failure. The indication of the underlying heart disease was ischaemic in 26 and non-ischaemic in 49 patients. The mean baseline NYHA functional class was 3.0 ± 0.7, left ventricular (LV) ejection fraction 35 ± 11%, and QRS duration 154 ± 29 ms. Sinus rhythm was present in 60, and atrial fibrillation (AF) in 15 patients. No patient had any significant heart valve disease. The mitral regurgitation was characterized by a regurgitant orifice area ≤ 20 mm2. The registry was reviewed and approved by the Ethics Review Committees of all participating medical centres, in compliance with the French national laws and regulations, and all patients were informed of, and had granted their informed consent to, their participation in the study.
sonR sensor
The sonR signal is measured by a piezoelectric micro-accelerometer sensor encapsulated in a hermetic can. Endocardial acceleration vibrations, generated throughout the cardiac cycle by successive combinations of complex mechanical events transmitted throughout the heart and thorax,9,10 are measurable either inside the myocardium or transcutaneously. SonR1 and sonR2, the main vibratory components observed during the cardiac cycle, correlate with the first and second heart sounds, respectively. SonR1, which starts at the onset of LV isovolumic contraction, is the combined product of AV valve closure, semilunar valve opening, LV contraction, and blood flow across the heart chambers. SonR2 is generated at the end of systole, by the closure of the semilunar valves. Previous studies have correlated the amplitude of sonR1 and LV dP/dtmax under various conditions.9–11
Recording procedure
A Biopac MP35 data acquisition system (Biopac Systems, Inc. Goleta, CA, USA) was used to simultaneously record at a 10 kHz sampling frequency (i) surface sonR filtered between 15 and 150 Hz, using a sensor clipped onto a standard surface electrocardiogram electrode in the parasternal region, (ii) 2-lead electrocardiogram, and (iii) Doppler signal, recorded from the audio output of a Vivid 7 ultrasound system (GE Healthcare, Horten, Norway).
According to the protocol (identically in every centre), different CRT modes were randomly selected, including biventricular stimulation with various VV delays (−40, −24, + 24, and + 40 ms), right and LV stimulation, and spontaneous rhythm, when appropriate.
The sonR signals were processed off-line, using custom-built Matlab® signal processing routines (The MathWorks, Inc., Natick, MA, USA), and the ultrasound recordings were analysed at the core laboratory of Rennes University ‘Centre d'Investigation Clinique et d'Innovation Technologique’.
For each record in a given stimulation configuration, the timing of the QRS or pacing artefact was automatically detected. This timing was used as a reference to divide the sonR and Doppler-echocardiographic signals into individual cardiac cycles.
Acquisition of surface sonR timings
We averaged separately 15 cardiac cycles of sonR1 and sonR2 recorded at the time of pulsed-wave Doppler recording. After high-pass filtering, an envelope was computed on the averaged sonR cycle, and a threshold-based segmentation algorithm was independently applied on sonR1 and sonR2, which derived an estimate of their onset (tsonRx_onset) and end (tsonRx_end). An example of sonR average cycle processing is shown in Figure 1. When excessive motion or environmental artefacts interfered with the quality of the external sonR signal, the record was rejected from analysis.
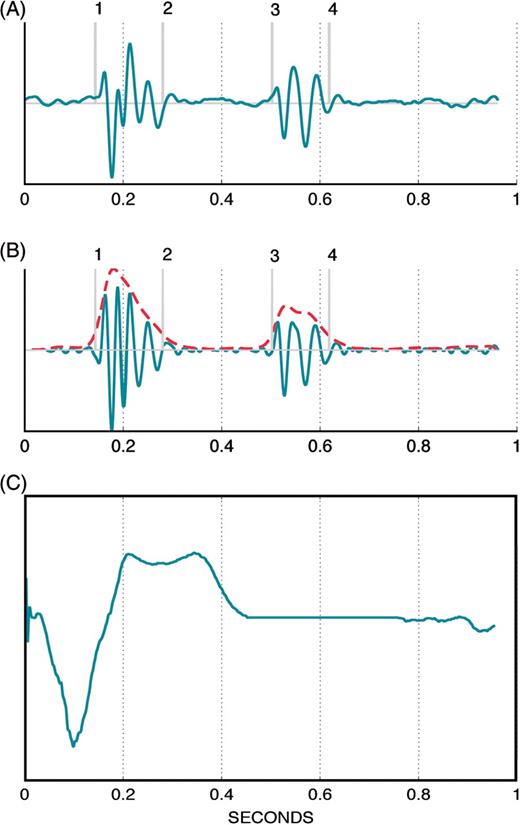
(A) Representative example of an average sonR cycle composed of 15 consecutive, ensemble-averaged cycles (B). The same average sonR cycle, high-pass filtered, and its envelope in doted gray line. The vertical markers are the detected components of sonR1 and sonR2, including, consecutively (1) tsonR1_onset, (2) tsonR1_end, (3) tsonR2_onset, and (4) tsonR2_end. t = 0, the onset of the cardiac cycle, is the pacing artefact or the QRS onset marker. (C) Average electrocardiographic signal composed of 15 consecutive, ensemble-averaged cycles.
Assisted measurements of echocardiographic events
To assure the best synchrony between the acquired electrocardiogram signals and the measured echocardiographic events, pulsed-wave Doppler profiles of the mitral valve inflow and LV outflow were obtained by applying standard signal-processing methods to the audio signals acquired from the Vivid 7. A custom-built platform (Figure 2) was then used by a trained operator to (i) display the obtained pulse wave doppler profile and (ii) manually place markers of mitral (tMC) and aortic (tAC) valves closures over three to six selected cycles, and calculate their average value. The duration of systole was calculated as the interval between tMC and tAC, while that of diastole was calculated as the RR interval minus the systolic duration. The previous step of automatic detection of QRS onsets or pacing spikes, still very simple from a signal processing point of view, significantly enhanced the reproducibility and precision of the echocardiographic markers. In addition, an independent echocardiographer validated these measurements.
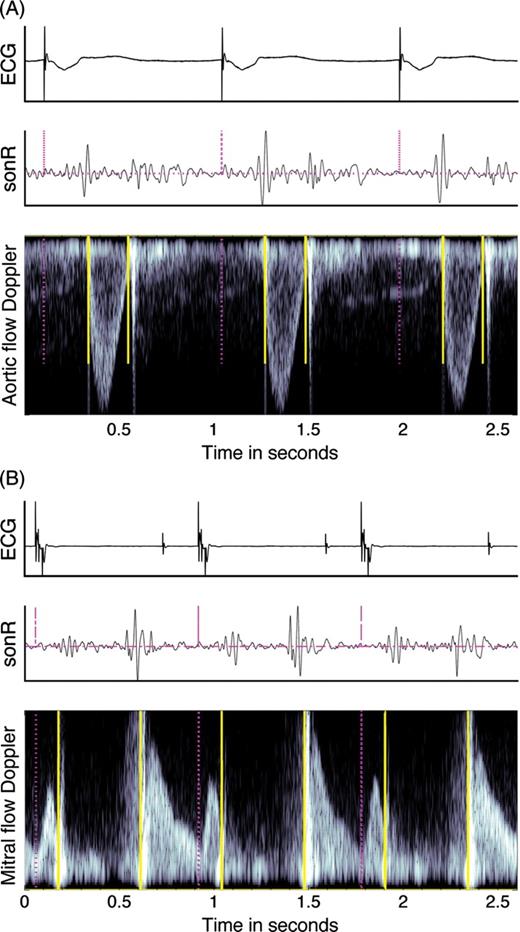
Example of markers on the aortic valve (A) and the mitral valve (B) pulsed wave Doppler. The pacing artefacts or QRS onset (dashed vertical lines) were automatically detected on the electrocardiogram (ECG); the sonR and the Doppler recordings were then segmented into individual cardiac cycles. The solid vertical lines mark the openings and closures of the aortic and mitral valves on the respective flow Doppler recordings.
A comparison of this approach with respect to the completely manual procedure performed with the EchoPac system (GE Healthcare, Horten, Norway) in 10 randomly selected patients (extrapolated for the population of the study) has shown that the measurements variability, calculated as the absolute difference divided by the average of the two measurements, was lower in our tool. Indeed, the variability was 18 ± 18% for tMC, and 2.5 ± 2.5% for tAC measured with EchoPac, while it was 5 ± 6% for tMC and 1 ± 1% for tAC when measured with our custom-built platform. That was performed by two persons in the dedicated Rennes University Hospital's core lab for echocardiography.
Statistical analysis
Correlations between echocardiographic and sonR-derived measurements were tested by linear regression analysis, where tsonR1_onset was the estimated onset of mitral closure, and tsonR2_onset was the estimated onset of aortic valve closure. The linear model applied for the estimation of the duration of systole was defined as a function of both tsonR1_onset and tsonR2_onset. The duration of diastole was estimated by a linear model including tsonR1_onset, tsonR2_onset, and the RR interval.
A correlation coefficient r was calculated to evaluate the quality of the linear regression. In addition, the average absolute error between sonR and ultrasound-derived measurements (µerr), was computed for each model. The quality of the regression was quantified by the correlation coefficient r,and average absolute error µerr, i.e. the average error between sonR and ultrasound-derived measurements. We considered that this method accurately estimated the two gold standard timings in individual patients when rpatient was >0.66 and µerr_patient was <20 ms.
Results
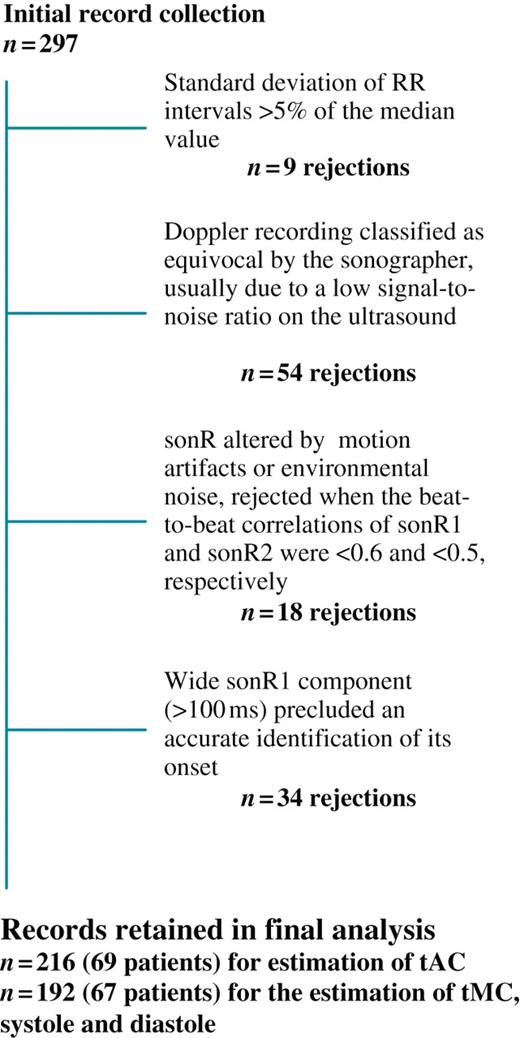
A total of 297 records (4 ± 1 per patient) were initially entered in the database (Table 1; Figure 3). The number of records excluded from the database for ≥1 criterion, and the specific criteria prompting their rejections are shown in Figure 3. A total of 216 records from 69 patients was ultimately available for the estimation of tAC, and 192 records from 67 patients for the estimation of tMC, systole and diastole. We did not observe any difference in ischaemic vs. non-ischaemic aetiology of the cardiomyopathy. Mitral regurgitation or other valvular diseases did not affect the quality of the sonR recordings.
Concordance between ultrasound and sonR-derived timings of mitral and aortic valve closure, and duration of systole and diastole
| Ultrasound . | sonR . | µerr . | r . | Concordance n (%) of patients . |
|---|---|---|---|---|
| tMC (mitral valve closure time) | tsonR1_onset | 9.7 ms | 0.86* | 63/67 (94) |
| tAC (aortic valve closure time) | tsonR2_onset | 9.7 ms | 0.93* | 65/69 (94) |
| Systole duration | tsonR1_onset and tsonR2_onset | 13.4 ms | 0.85* | 54/67 (81) |
| Diastole duration | RR, tsonR1_onset and tsonR2_onset | 12.0 ms | 0.99* | 55/67 (82) |
| Ultrasound . | sonR . | µerr . | r . | Concordance n (%) of patients . |
|---|---|---|---|---|
| tMC (mitral valve closure time) | tsonR1_onset | 9.7 ms | 0.86* | 63/67 (94) |
| tAC (aortic valve closure time) | tsonR2_onset | 9.7 ms | 0.93* | 65/69 (94) |
| Systole duration | tsonR1_onset and tsonR2_onset | 13.4 ms | 0.85* | 54/67 (81) |
| Diastole duration | RR, tsonR1_onset and tsonR2_onset | 12.0 ms | 0.99* | 55/67 (82) |
tMC, time to mitral valve closure; tAC, time to aortic valve closure derived an estimate of sonR1 and sonR2 onset (tsonR1 or 2_onset) and end (tsonR1 or 2_end); r, correlation coefficient; ms, millisecond; µerr, average absolute error.
*P < 0.0001.
Concordance between ultrasound and sonR-derived timings of mitral and aortic valve closure, and duration of systole and diastole
| Ultrasound . | sonR . | µerr . | r . | Concordance n (%) of patients . |
|---|---|---|---|---|
| tMC (mitral valve closure time) | tsonR1_onset | 9.7 ms | 0.86* | 63/67 (94) |
| tAC (aortic valve closure time) | tsonR2_onset | 9.7 ms | 0.93* | 65/69 (94) |
| Systole duration | tsonR1_onset and tsonR2_onset | 13.4 ms | 0.85* | 54/67 (81) |
| Diastole duration | RR, tsonR1_onset and tsonR2_onset | 12.0 ms | 0.99* | 55/67 (82) |
| Ultrasound . | sonR . | µerr . | r . | Concordance n (%) of patients . |
|---|---|---|---|---|
| tMC (mitral valve closure time) | tsonR1_onset | 9.7 ms | 0.86* | 63/67 (94) |
| tAC (aortic valve closure time) | tsonR2_onset | 9.7 ms | 0.93* | 65/69 (94) |
| Systole duration | tsonR1_onset and tsonR2_onset | 13.4 ms | 0.85* | 54/67 (81) |
| Diastole duration | RR, tsonR1_onset and tsonR2_onset | 12.0 ms | 0.99* | 55/67 (82) |
tMC, time to mitral valve closure; tAC, time to aortic valve closure derived an estimate of sonR1 and sonR2 onset (tsonR1 or 2_onset) and end (tsonR1 or 2_end); r, correlation coefficient; ms, millisecond; µerr, average absolute error.
*P < 0.0001.
Numbers and causes of rejections of records from final analysis. Note that ≥1 criterion may have applied to a single record.
Timings of aortic and mitral valve closure
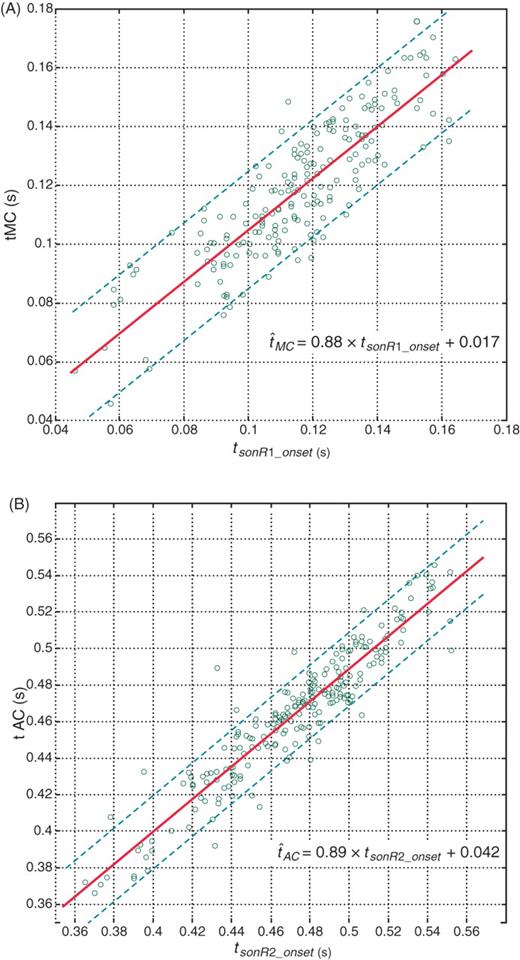
Figure 4 shows the linear regression between (i) tMC and tsonR1_onset, and (ii) tAC and tsonR2_onset; the main study results are summarized in Table 1. High correlations were found between tMC and tsonR1_onset (r = 0.86; µerr = 9.7 ms), as well as between tAC and tsonR2_onset (r = 0.93; µerr = 9.7 ms). The timing of mitral valve closure estimated by ultrasound vs. sonR was concordant in 65 of 69 (94%) patients, and that of aortic valve closure in 63 of 67 (94%) patients (Table 1).
Correlation (A) between the sonR1 onset timing and timing of mitral valve closure (tMC) measured by echo and (B) between the sonR2 onset and the timing of aortic valve closing (tAC). Each point corresponds to one record. The regression line is shown as a solid line, and the two parallel dashed lines are the 20 ms error limits.
In separate regression analyses of 15 patients with, vs. 52 patients without AF, no difference was found between the two groups. For the timing of mitral valve closure, the qualitative regression indicators were r = 0.73 and µerr = 9.3 ms in the AF group, and r = 0.87 and µerr= 9.6 ms in the non-AF group, compared with r = 0.86 and µerr = 9.7 in the entire population. Corresponding regression indicators for the timing of aortic valve closure were r = 0.85 and µerr = 11.2 ms in the AF group and r = 0.94 and µerr = 9.3 ms in the non-AF group, compared with r = 0.93 and µerr = 9.7 ms in the entire population. Moreover, of four patients with discordant tMC measured by ultrasound vs. sonR, two were in AF, while none of the for patients with discordant tAC measured by ultrasound vs. sonR were in AF. It is noteworthy that the number of AF records was low, and that the ranges of variations of the two echocardiographic timings (100–160 ms for tMC, and 400–500 ms for tAC, vs. 50–165 ms and 360–550 ms, respectively, in the entire population) were narrow, which alone might explain the slightly lower quality of the regression in the AF group.
Durations of systole and diastole
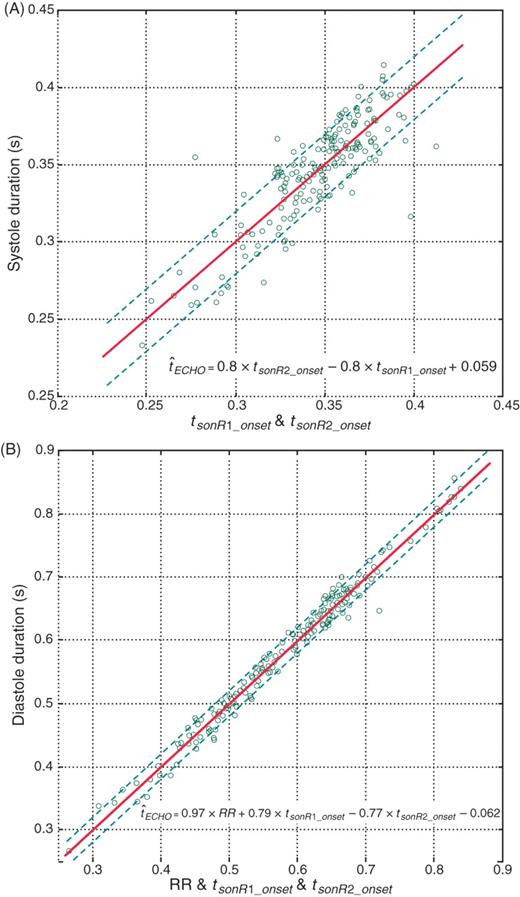
The duration of systole was calculated as the interval between tMC and tAC, while that of diastole was calculated as the RR interval minus the systolic duration. Figure 5 shows the linear regression between sonR and ultrasound in the estimation of systolic (A) and diastolic (B) duration. The correlation of the two methods (r = 0.85, µerr = 13.4 ms for systole, and r = 0.99, µerr = 12 ms for diastole) was high (Table 1), and a high concordance was observed in 55 out of 67 (81%) records.
Correlation between sonR timings and the echo-derived (A) systolic and (B) diastolic durations. We plotted the sonR-estimated systole and diastole vs. the reference ultrasound measurement. The line of identity is shown as a solid line.
Discussion
This study is the first to show a close concordance between sonR and cardiac ultrasound in the timing of aortic and mitral valves closure, using standard signal-processing techniques. The timing of closure of both valves was accurately detected by sonR in >90% of patients. The proportions of patients satisfying our criteria for the duration of systole (81%) and diastole (82%) were slightly lower. This is explained by the additive effect of the number of inaccurate and independent measurements of tMC and tAC, which, when combined, lowered the proportion of accurately estimated durations of systole and diastole. If our observations are confirmed, this technology would be interesting to test for an automatic and adaptive optimization of implantable CRT systems, and might therefore lower the rate of non-response to CRT.
Validation of the concept
Although it has not been widely confirmed, the optimization of stimulation settings is expected to lower the proportion of non-responders to CRT.12 The individual optimization of AV and VV delays is important, since considerable inter-individual variations have been observed, including in non-failing hearts with left bundle branch block.12 This optimization procedure has been included in randomized clinical trials,3,4 and is currently carried out at rest, using echocardiography, usually before discharge of the patient from the hospital after CRT system implantation, and repeated later if adjustments in the device programming do not improve the patient's clinical status. Using ultrasound imaging of mitral and aortic flows, the feasibility and reproducibility of timing and measurements of systolic and diastolic events are not 100%. Echocardiographic optimization of CRT is time consuming and underused in clinical practice. Artefacts or improper Doppler alignment may lower the quality of the measurements. In the present study, we used custom-developed Doppler echocardiographic instrumentation to minimize the effects of these potential methodologic limitations, and found a clinically important correlation between the echocardiographic and the sonR timings of cardiac mechanical events. To the best of our knowledge, an implantable system to monitor cardiac mechanical events, valve closure in particular, has not been firmly validated.13,14
Clinical applications
The phases of the cardiac cycle are usually altered by chronic heart failure. In the presence of a left bundle branch block, the isovolumic intervals are prolonged and the LV diastolic filling period is shortened.15 Because of the various ventricular activation patterns associated with LV vs. biventricular pacing, as well as various AV delays, causing variable degrees of fusion between stimulated and natural wavefronts, marked differences in haemodynamic results can be expected with programming with different devices. A simple optimization method based on the pathophysiology of heart failure and ventricular dyssynchrony is needed. Invasive and non-invasive methods have been implemented, which mostly used indices of LV systolic function, notably LV dP/dtmax and arterial pulse pressure.16 These optimization methods have the disadvantages of being time and resource consuming, poorly reproducible, and dependent on a high image quality and highly experienced operators. Therefore, an alternative, operator-independent, inexpensive system, which can be included in an implantable CRT pulse generator, was developed. sonR, which can be incorporated in ventricular or atrial endocardial leads,17 might enable the automatic programming of cardiac stimulation, and provide the physicians with a means of optimizing ventricular filling and systolic function. Other variables that warrant attempts at optimization include the left pre-ejection interval, the left pre-ejection/ejection duration ratio, or both.18 The capability of this new opportunity for optimizing the pacing mode automatically would have to be evaluated as previous experience with other approaches did not demonstrate any beneficial effect (QuickOpt, Expert Ease…).19 QT interval dynamics has recently been proposed and demonstrated to be closely correlated with changes in ejection time, thus constituting an electrical parameter of systolic time.20
Limitations of the study
Despite these positive results, we observed a few instances of poor correlation between ultrasound and sonR markers. The clinical characteristics of these patients were not significantly different from the rest of the population. Moreover, a sonR1 component wider than 100 ms, observed in 34 out of 297 records, precluded the accurate detection of the onset of sonR1. This cause of data rejection will be examined in greater depth in future studies. A better understanding of the complex relationship between mechanical and haemodynamic events and sonR is needed to explain these cases. Secondly, we used echocardiography as a reference method to optimize the CRT systems, despite its known limitations. However, we significantly enhanced the reliability of this reference with our custom-built Doppler audio signal-processing platform. Thirdly, our recordings were performed at rest, with haemodynamic changes induced by changes in the stimulation configuration. Our method needs to be evaluated further, under broader conditions and during changes in metabolic demand induced by pharmacologic interventions or exercise. Finally, we tested an external prototype of the sonR sensor, exposed to environmental noise, from patient motion, cough, speech, echocardiographic probe, and others, against which an implantable system should be protected.
Conclusions
This study, which shows the potential contributions of sonR timings in the detection of cardiac mechanical events, and in the precise measurement of systolic and diastolic periods, suggests that this sensor could be used in the monitoring of cardiac function and adaptive optimization of CRT.
Funding
This study was sponsored by Sorin Group/Ela Medical, and supported, in part, by a grant from the French National Research Agency (ANR-08-TECS013).
Acknowledgments
The authors thank Pascal Lotton, Kamilia Djouadi, Mélanie Heurteau, and Laurence Graindorge for their contributions to this study, and Rodolphe Ruffy, MD (www.cardioscript.com) for his assistance in the preparation of the manuscript.
Conflict of interest: M.B., S.C., E.D., A.H., G.J., C.L., and P.M. have received consulting honoraria from Sorin CRM SAS.
References
- aorta
- aortic valve
- electrocardiogram
- left ventricular ejection fraction
- echocardiography
- ultrasonography
- mitral valve
- endocardium
- doppler echocardiography
- diastole
- systole
- heart
- microbiology procedures
- cardiac resynchronization therapy
- cardiac function
- cardiac cycle
- qrs complex duration
- linear regression
- accelerometers
- sensor